Bowel Resection and Anastomosis
Dimitri Alexander Petrov, MD
The Operative Review of Surgery. 2023; 1:232-242.
Table of Contents
Bowel Resection
Types of Resection
- Esophagectomy: Resection of the Esophagus
- Gastrectomy: Resection of the Stomach
- Small Bowel Resection (SBR): Resection of the Small Bowel
- Colectomy: Resection of the Colon
- Proctectomy: Resection of the Rectum
General Principles
- Ensure Good Blood Supply – Judge Subjectively or Use Doppler/Scintigraphy
- Ensure Adequate Mobilization and Avoid Tension
- Angle Transection Line Straight or Somewhat Oblique to Keep Antimesenteric Edge Shorter and Ensure Adequate Blood Supply
Classic Technique
- Place Non-Crushing Bowel Clamps on Both Sides of the Transection Line
- Sharply Transect the Bowel Between the Clamps Using a Scalpel
- Repeat at the Opposite Bowel Margin
- Transect the Mesentery Using an Electrosurgical Device or by Clamping and Tying
- Remove Specimen
- Perform Anastomosis
Linear Stapler Technique (Most Common)
- Pinch and Thin the Mesentery Just Under the Site of Transection
- Make a Small Mesenteric Defect at the Site
- Insert One Jaw of the Stapler Through the Defect
- Assemble & Fire the Stapler at the Transection Line
- Repeat at the Opposite Bowel Margin
- Transect the Mesentery Using an Electrosurgical Device or by Clamping and Tying
- Remove Specimen
- Perform Anastomosis
Bowel Anastomosis
General Principles
- “Strength Layer” of Bowel: Submucosa 1
- Ensure that the Submucosa is Always Included in the Anastomosis
- All Layers Add to the Strength
- Antimesenteric Border is at the Highest Risk for Ischemia – The Vasa Recta Arise from the Most Peripheral Arcades and Do Not Intercommunicate 2
- Center Side Reconstructions On:
- Small Bowel: Antimesenteric Border (Limit Ischemia)
- Large Bowel: Taenia Coli (Adds Strength)
- Anastomosis Weakest Time Point: 3-5 Days (Breakdown Exceeds Production) 3
- Bowel Has Increased Collagenase Activity Compared to Skin
Type of Anastomosis
- End-to-End
- The End of One Loop is Anastomosed to the End of Another Loop
- More “Physiologic” in Replication of Normal Gut Motility
- End-to-Side
- The End of One Loop is Anastomosed to the Side of Another Loop
- Generally Considered When There is a Size Mismatch (Small Bowel to Colon, etc.)
- Side-to-Side
- Bowel Loops are Oriented Side-to-Side in an Overlapping Fashion
- The Most Common Technique when Using a Linear Stapler
- Types:
- Isoperistaltic: Ends are Approximated to Maintain Similar Directions of Peristalsis
- Technically More Difficult
- Antiperistaltic: Ends are Approximated with Opposite Directions of Peristalsis
- Technically Less Difficult
- Often Referred to as “Functional End-to-End”
- Isoperistaltic: Ends are Approximated to Maintain Similar Directions of Peristalsis
Intraoperative Assessment of Anastomosis Perfusion/Viability
- Subjective Findings:
- Bowel Color
- Observed Pulsatile Blood Flow at the Transected Section
- Objective Findings:
- Indocyanine Green Fluorescence Angiography (ICG-FA/ICGA) – Best Studied
- *See Below
- Doppler US – Minimal Data
- Light Spectroscopy – Minimal Data
- Indocyanine Green Fluorescence Angiography (ICG-FA/ICGA) – Best Studied
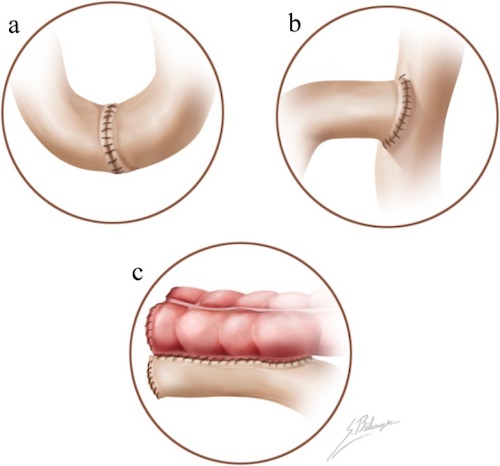
Anastomosis; (a) End-to-End, (b) End-to-Side, (c) Side-to-Side 4
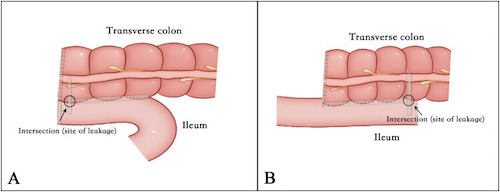
Side-to-Side Anastomosis; (A) Antiperistaltic, (B) Isoperistaltic 5
Hand-Sewn vs Stapled Techniques
Hand-Sewn Technique
- Excise Staple Lines if Present
- Use a Stay Suture to Approximate Both Bowel Ends
- Create Anastomosis with Full-Thickness Bites Using Absorbable Suture – Techniques are Varied (Interrupted vs Running/Direction of Travel)
- Consider Seromuscular Lembert Sutures to Buttress as a Second Layer
- Close the Mesenteric Defect
Linear Stapled Anastomosis (Antiperistaltic Side-to-Side)
- Traditional Method:
- Resect the Proximal and Distal Ends
- Sharply Excise the Antimesenteric Corner of the Resected Ends
- Place the Two Jaws of the Linear Stapler Through Each Opening
- Arrange the Jaws Along the Antimesenteric Borders
- Assemble and Fire the Stapler to Create a Common Channel
- Consider Placing a Reinforcing Silk Stitch (“Crotch Stich”) at the Inner Junction – The Site of Most Tension
- Close the Enterotomies – Either with Suture or With Another Staple Load
- Close the Mesenteric Defect
- Barcelona Technique:
- Approximate the Proximal and Distal Ends (Prior to Resection)
- Make Small Antimesenteric Enterotomies at the Anticipated Ends
- Place the Two Jaws of the Linear Stapler Through Each Opening
- Arrange the Jaws Along the Antimesenteric Borders
- Assemble and Fire the Stapler to Create a Common Channel
- Use Another Stapler to Amputate the Specimen, Including the Previous Enterotomies
- Resect the Specimen and Close the Mesenteric Defect
- *Benefits: Cost-Effective and Only Uses Two Staple Loads (Traditional Uses 4 Staple Loads)
End-to-End Stapled Anastomosis (EEA)
- *Generally Used for Colorectal or Upper GI Anastomoses
- Complete the Indicated Bowel Resection
- The Suture Line of the Proximal End is Resected
- A Purse-String Suture is Used to Close the Lumen Around an Anvil
- Can Also Be Brought Out of the Side for an End-to-Side Anastomosis
- An Assistant Advances the EEA Stapler Through the Anus and Positions Appropriately
- The Stapler is Opened, Piercing a Guidepost Through the Distal Bowel Wall
- The Anvil is Attached to the Guidepost
- The Stapler is then Closed and Fired
- The Stapler is then Removed Through the Anus and Inspected to Confirm the Presence of Two Rings (“Donuts”) Implying a Complete Anastomosis
- Consider Performing a Leak Test
- The Pelvis is Filled with Saline and the Proximal End of the Anastomoses Bowel is Occluded
- Air is Gently Insufflated Through the Rectum
- The Pelvis is Inspected for Air Bubbles That Would Indicate a Leak
- Small Leaks Can Be Repaired Primarily
- Large Leaks Should Be Taken Down with Repeat Anastomosis
Comparison
- No Significant Difference in Outcomes Between the Various Techniques 6,7
- Single-Layer vs Double-Layer Hand-Sewn Anastomoses: 8,9
- No Difference in Anastomotic Leak Rates
- Single-Layer are Quicker to Perform
- Hand-Sewn vs Stapled Anastomoses: 7,10,11
- No Difference in Anastomotic Leak Rates
- There are Some Conflicting Data with Strong Views Either Way
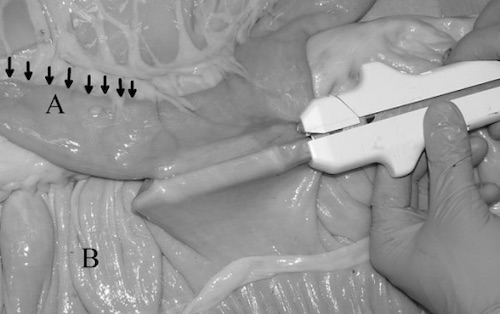
Side-to-Side Stapled Anastomosis 12
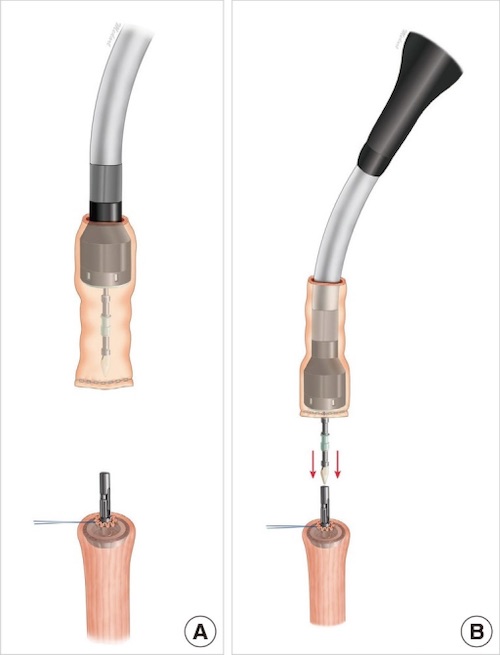
End-to-End Stapled Anastomosis 13
Indocyanine Green Fluorescence Angiography (ICG-FA/ICGA)
Mechanism/Theory
- Indocyanine Green (ICG) is a Fluorescent Probe in Response to Near-Infrared (NIR) Light
- Binds Primarily to Serum Albumin and Other Plasma Proteins
- Primarily Confined to the Intravascular Compartment with Minimal Leakage
- Excreted Almost Exclusively into Bile
- Negligible Toxicity
Administration
- Timing:
- Bowel Should Enhance < 60 Seconds
- Half-Life: 3-5 Minutes
- Cleared by Liver: 15-20 Minutes
- Dosing: Poorly Standardized
- 2-0.5 mg/kg
- 5-12.5 mg
Imaging Systems
- Firefly (Intuitive Surgical)
- SPY Elite (Stryker)
- PINPOINT (Stryker)
- IMAGE1 S (Karl Storz)
- D-LIGHT P SCB (Karl Storz)
Outcomes
- Colorectal Anastomoses: Possibly Decreased Risk of Anastomotic Leak, Reoperation, and Overall Complications (Debated) 14,15
- Small Bowel Anastomoses: Insufficient Data
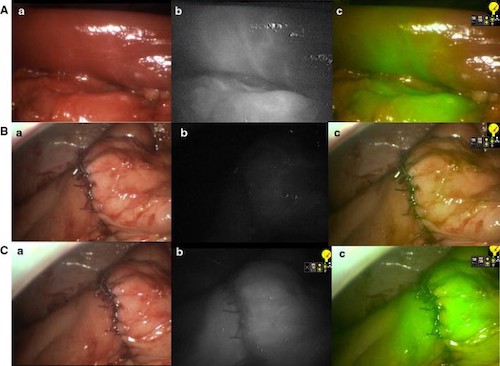
Right Hemicolectomy by ICG-FA; (A) Bowel After Vessel Division with Demarcation, (B) Anastomosis Before ICG, (C) Anastomosis After ICG; (a) Normal Light, (b) NIR, (c) Superimposition of NIR in Green 16
Anastomotic Leak
Leak Rates 17
- Overall: 2-7%
- Ileocolic: 1-3% (Lowest)
- Colocolic: 6-12%
- Coloanal: 10-20% (Highest)
Risk Factors 18-22
- Patient Factors:
- Male Sex
- Malnutrition (Low Albumin)
- Obesity
- High ASA Score
- Operative Factors:
- Emergency Surgery (Compared to Elective)
- Prolonger Operative Time (> 4 Hours)
- Ischemia/Tension
- Tumor Size > 5 cm
- Multiple Stapler Firings
- Low Anastomosis (< 5 cm from the Anal Verge)
- Lateral LN Dissection
- Debated Risk Factors:
- NSAIDs/Ketorolac (Toradol)
- Corticosteroids
- Drains
Presentation
- Generally Present at 5-7 Days Postoperatively
- Abdominal Pain
- Fever
- Tachycardia
- Peritonitis
- Purulent or Feculent Drainage
- May Present with an Abscess or Gas/Fluid Collection
Complications of Anastomotic Leak
- Increased Mortality (15-30% vs 2-4%) 23-25
- Prolonged Hospital Stay 24
- Increased Risk of Cancer Recurrence 26,27
- Chronic Presacral Sinus 28
- Stricture
Diagnosis
- Most Often Made by CT (Triple Contrast – PO, IV, and Rectal) 29
- Consider Surgical Exploration if Unstable or Peritoneal
- Other Options: Contrast Enema or Endoscopy 29
Treatment 30,31
- Subclinical/Radiographic: Expectant Management
- Small (< 3 cm) Contained Abscess: Expectant Management and Antibiotics
- Large (> 3 cm) or Multiloculated Abscess: Percutaneous Drain
- If Not Feasible or Fails: Surgical Drainage
- Unstable, Peritonitis, or Free Intraperitoneal Leak: Surgical Repair
- Major Defects (> 1 cm or One-Third Circumference) Require Resection with Either Revision or Diversion
Other Complications
Bleeding
- Definitions:
- Minor Bleeding: Bleeding that Does Not Require Transfusion or Intervention
- Major Bleeding: Bleeding that Causes Hemodynamic Instability or Requires Transfusion or Intervention (0.5-4.2%)
- Minor Bleeding is Common and Most Often Stops within 24-48 Hours
- 50% Will Progress to Major Bleeding 32
- Treatment: 33-35
- Initial Management: Supportive Care and Blood Transfusion
- Persistent Bleeding Requires Endoscopic Intervention
- May Consider Transanal Operative Interventions for a Low Anastomosis
- May Consider Angiographic Embolization (Theoretic Risk of Ischemia/Dehiscence)
- Indications for Surgical Intervention:
- Early Hemodynamic Instability That Does Not Respond to Aggressive Resuscitation
- Failure of Endoscopic Management
Stricture
- Incidence:
- Overall: 3-30% 36,37
- Clinically Significant Stricture: 4-10%
- Risk Factors: Ischemia, Inflammation, Radiation, Leak, or Recurrent Disease 37
- Stricture After Resection of Malignancy Requires Evaluation of Potential Local Recurrence (CEA, CT, EUS, etc.)
- General Treatment of Benign Strictures: Repeated Endoscopic Dilation
- Success Rate: 88-100% 37,38
- Indications for Surgical Revision:
- Malignant Strictures without Distant Metastatic Disease
- Refractory Strictures After Repeated Endoscopic Dilations
Enteric Fistula
- Incidence: 1-10% 39-42
- Sites: Skin, Bladder, Vagina, or Presacral Space
- *See Enteric Fistula
References
- Bosmans JW, Jongen AC, Bouvy ND, Derikx JP. Colorectal anastomotic healing: why the biological processes that lead to anastomotic leakage should be revealed prior to conducting intervention studies. BMC Gastroenterol. 2015 Dec 21;15:180.
- Cho KJ, Varma MK. Mesenteric Vascular Disease. In: Current Therapy in Vascular and Endovascular Surgery. Elsevier. 2014.
- Tang Z, Cai H, Cui Y. Influence of Early Postoperative Feeding in Gastrointestinal Anastomotic Fistula Formation and Healing Time in Rabbits. Biomed Res Int. 2018 May 6;2018:8258096.
- Terrone DG, Lepanto L, Billiard JS, Olivié D, Murphy-Lavallée J, Vandenbroucke F, Tang A. A primer to common major gastrointestinal post-surgical anatomy on CT-a pictorial review. Insights Imaging. 2011 Dec;2(6):631-638. (License: CC BY 2.0)
- Zhang M, Lu Z, Hu X, Zhou H, Zheng Z, Liu Z, Wang X. Comparison of the short-term outcomes between intracorporeal isoperistaltic and antiperistaltic totally stapled side-to-side anastomosis for right colectomy: A retrospective study on 214 consecutive patients. Surg Open Sci. 2022 Mar 26;9:7-12. (License: CC BY-NC-ND 4.0)
- Slieker JC, Daams F, Mulder IM, Jeekel J, Lange JF. Systematic review of the technique of colorectal anastomosis. JAMA Surg. 2013 Feb;148(2):190-201.
- Bruns BR, Morris DS, Zielinski M, Mowery NT, Miller PR, Arnold K, Phelan HA, Murry J, Turay D, Fam J, Oh JS, Gunter OL, Enniss T, Love JD, Skarupa D, Benns M, Fathalizadeh A, Leung PS, Carrick MM, Jewett B, Sakran J, O’Meara L, Herrera AV, Chen H, Scalea TM, Diaz JJ. Stapled versus hand-sewn: A prospective emergency surgery study. An American Association for the Surgery of Trauma multi-institutional study. J Trauma Acute Care Surg. 2017 Mar;82(3):435-443.
- Sajid MS, Siddiqui MR, Baig MK. Single layer versus double layer suture anastomosis of the gastrointestinal tract. Cochrane Database Syst Rev. 2012 Jan 18;1:CD005477.
- Shikata S, Yamagishi H, Taji Y, Shimada T, Noguchi Y. Single- versus two- layer intestinal anastomosis: a meta-analysis of randomized controlled trials. BMC Surg. 2006 Jan 27;6:2.
- Suturing or stapling in gastrointestinal surgery: a prospective randomized study. West of Scotland and Highland Anastomosis Study Group. Br J Surg. 1991 Mar;78(3):337-41.
- Choy PY, Bissett IP, Docherty JG, Parry BR, Merrie A, Fitzgerald A. Stapled versus handsewn methods for ileocolic anastomoses. Cochrane Database Syst Rev. 2011 Sep 7;(9):CD004320.
- Gandini M, Giusto G, Iotti B, Valazza A, Sammartano F. In vitro description of a new technique for stapled side-to-side jejunocecal anastomosis in horses and CT scan anatomical comparison with other techniques. BMC Vet Res. 2014;10 Suppl 1(Suppl 1):S9. (License: CC BY 4.0)
- Park J, Kang N, Oh SH, Lee SI, Song SH. The Use of a Mechanical Stapler in Jejunal Free Flaps in Laryngopharyngectomy Defects. Arch Plast Surg. 2015 Nov;42(6):815-8. (License: CC BY-NC 3.0)
- Morales-Conde S, Alarcón I, Yang T, Licardie E, Camacho V, Aguilar Del Castillo F, Balla A. Fluorescence angiography with indocyanine green (ICG) to evaluate anastomosis in colorectal surgery: where does it have more value? Surg Endosc. 2020 Sep;34(9):3897-3907.
- Tang G, Du D, Tao J, Wei Z. Effect of Indocyanine Green Fluorescence Angiography on Anastomotic Leakage in Patients Undergoing Colorectal Surgery: A Meta-Analysis of Randomized Controlled Trials and Propensity-Score-Matched Studies. Front Surg. 2022 Mar 15;9:815753.
- Ris F, Hompes R, Cunningham C, Lindsey I, Guy R, Jones O, George B, Cahill RA, Mortensen NJ. Near-infrared (NIR) perfusion angiography in minimally invasive colorectal surgery. Surg Endosc. 2014 Jul;28(7):2221-6. (License: CC BY 4.0)
- Dietz DW, Bailey HR. Postoperative complications. In: ASCRS Textbook of Colon and Rectal Surgery, Church JM, Beck DE, Wolff BG, et al (Eds), Springer-Verlag New York, LLC, New York 2006.
- Awad S, El-Rahman AIA, Abbas A, Althobaiti W, Alfaran S, Alghamdi S, Alharthi S, Alsubaie K, Ghedan S, Alharthi R, Asiri M, Alzahrani A, Alotaibi N, Shoma A, Sheishaa MSA. The assessment of perioperative risk factors of anastomotic leakage after intestinal surgeries; a prospective study. BMC Surg. 2021 Jan 7;21(1):29.
- Alverdy JC, Schardey HM. Anastomotic Leak: Toward an Understanding of Its Root Causes. J Gastrointest Surg. 2021 Nov;25(11):2966-2975.
- Sciuto A, Merola G, De Palma GD, Sodo M, Pirozzi F, Bracale UM, Bracale U. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol. 2018 Jun 7;24(21):2247-2260.
- Vasiliu EC, Zarnescu NO, Costea R, Neagu S. Review of Risk Factors for Anastomotic Leakage in Colorectal Surgery. Chirurgia (Bucur). 2015 Jul-Aug;110(4):319-26.
- Platell C, Barwood N, Dorfmann G, Makin G. The incidence of anastomotic leaks in patients undergoing colorectal surgery. Colorectal Dis. 2007 Jan;9(1):71-9.
- Choi HK, Law WL, Ho JW. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: analysis of risk factors. Dis Colon Rectum. 2006 Nov;49(11):1719-25.
- Slieker JC, Komen N, Mannaerts GH, Karsten TM, Willemsen P, Murawska M, Jeekel J, Lange JF. Long-term and perioperative corticosteroids in anastomotic leakage: a prospective study of 259 left-sided colorectal anastomoses. Arch Surg. 2012 May;147(5):447-52.
- Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey EL. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004 Aug;240(2):255-9.
- Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011 May;253(5):890-9.
- Docherty JG, McGregor JR, Akyol AM, Murray GD, Galloway DJ. Comparison of manually constructed and stapled anastomoses in colorectal surgery. West of Scotland and Highland Anastomosis Study Group. Ann Surg. 1995 Feb;221(2):176-84.
- Arumainayagam N, Chadwick M, Roe A. The fate of anastomotic sinuses after total mesorectal excision for rectal cancer. Colorectal Dis. 2009 Mar;11(3):288-90.
- Gessler B, Eriksson O, Angenete E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int J Colorectal Dis. 2017 Apr;32(4):549-556.
- Chiarello MM, Fransvea P, Cariati M, Adams NJ, Bianchi V, Brisinda G. Anastomotic leakage in colorectal cancer surgery. Surg Oncol. 2022 Mar;40:101708.
- Blumetti J, Chaudhry V, Cintron JR, Park JJ, Marecik S, Harrison JL, Prasad LM, Abcarian H. Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World J Surg. 2014 Apr;38(4):985-91.
- Cirocco WC, Golub RW. Endoscopic treatment of postoperative hemorrhage from a stapled colorectal anastomosis. Am Surg. 1995 May;61(5):460-3.
- Martínez-Serrano MA, Parés D, Pera M, Pascual M, Courtier R, Egea MJ, Grande L. Management of lower gastrointestinal bleeding after colorectal resection and stapled anastomosis. Tech Coloproctol. 2009 Mar;13(1):49-53.
- Malik AH, East JE, Buchanan GN, Kennedy RH. Endoscopic haemostasis of staple-line haemorrhage following colorectal resection. Colorectal Dis. 2008 Jul;10(6):616-8.
- Golda T, Zerpa C, Kreisler E, Trenti L, Biondo S. Incidence and management of anastomotic bleeding after ileocolic anastomosis. Colorectal Dis. 2013;15(10):1301-8.
- Biraima M, Adamina M, Jost R, Breitenstein S, Soll C. Long-term results of endoscopic balloon dilation for treatment of colorectal anastomotic stenosis. Surg Endosc. 2016;30(10):4432–4437.
- Chan RH, Lin SC, Chen PC, Lin WT, Wu CH, Lee JC, Lin BW. Management of colorectal anastomotic stricture with multidiameter balloon dilation: long-term results. Tech Coloproctol. 2020 Dec;24(12):1271-1276.
- Hassan C, Zullo A, De Francesco V, et al. Systematic review: endoscopic dilatation in Crohn’s disease. Aliment Pharmacol Ther. 2007;26(11–12):1457–1464.
- Kosugi C, Saito N, Kimata Y, Ono M, Sugito M, Ito M, Sato K, Koda K, Miyazaki M. Rectovaginal fistulas after rectal cancer surgery: Incidence and operative repair by gluteal-fold flap repair. Surgery. 2005 Mar;137(3):329-36.
- Fazio VW, Zutshi M, Remzi FH, Parc Y, Ruppert R, Fürst A, Celebrezze J Jr, Galanduik S, Orangio G, Hyman N, Bokey L, Tiret E, Kirchdorfer B, Medich D, Tietze M, Hull T, Hammel J. A randomized multicenter trial to compare long-term functional outcome, quality of life, and complications of surgical procedures for low rectal cancers. Ann Surg. 2007 Sep;246(3):481-8; discussion 488-90.
- Matthiessen P, Hansson L, Sjödahl R, Rutegård J. Anastomotic-vaginal fistula (AVF) after anterior resection of the rectum for cancer–occurrence and risk factors. Colorectal Dis. 2010 Apr;12(4):351-7.
- Goriainov V, Miles AJ. Anastomotic leak rate and outcome for laparoscopic intra-corporeal stapled anastomosis. J Minim Access Surg. 2010 Jan;6(1):6-10.