Familial Adenomatous Polyposis (FAP)
Johnathan W. Cain, DO
The Operative Review of Surgery. 2023; 1:103-111.
Table of Contents
Pathophysiology
Genetic Mutation 1
- Mutation in the APC Gene
- APC: Adenomatous Polyposis Coli
- Tumor Suppressor Gene
- Autosomal Dominant
Incidence 2
- Incidence: 1/8,300 Births
- Accounts for < 1% of All Colorectal Cancer Cases
Presentation
- Hallmark is 100’s-1,000’s of Colorectal Polyps 3
- 100% Lifetime Risk of Colorectal Cancer
- 70-80% of Tumors Occur in the Left Colon 4
Histology
- Numerous Sessile Polyps Throughout the Colon
- Generally ≤ 1 cm
- Various Histologic Features, Similar to Sporadic Colorectal Cancers 5
- The Adenomas Themselves are More Abundant but Do Not Have an Individually Higher Risk of Malignancy
Timing and Progression
- Onset of Polyps: 6
- 50% by Age 15
- 95% by Age 35
- Average Age of Colorectal Cancer Diagnosis: 35-40 7
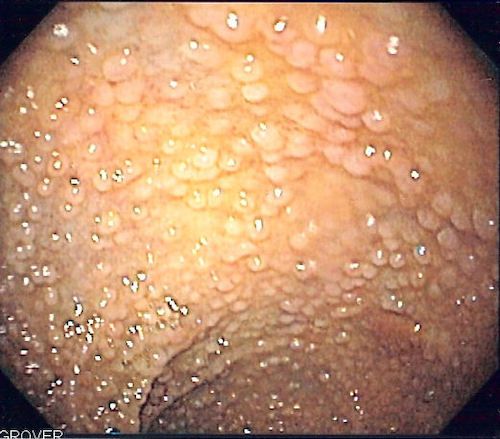
FAP on Colonoscopy 8
Extracolonic Manifestations
Duodenum Polyps
Stomach Polyps
- Incidence: 30-70% 3
- Second Most Common Site of Adenomas 3
- Second Most Common Cause of Death 9
- Predilection for the Ampulla and Periampullary Region 10
- Spigelman Staging of Duodenal Polyps in FAP 11
- Stage 0: 0 Points
- Stage I: 1-4 Points
- Stage II: 5-6 Points
- Stage III: 7-8 Points
- Stage IV: 9-12 Points
| Factor | 0 Points | 1 Point | 2 Points | 3 Points |
| Number of Polyps | 0 | 1-4 | 5-20 | > 20 |
| Polyp Size | No Polyps | 1-4 mm | 5-10 mm | > 10 mm |
| Histology | No Adenomas | Tubular Adenoma | Tubulovillous Adenoma | Villous Adenoma |
| Dysplasia | None | Low Grade | NA | High Grade |
- Incidence: 30-88% 3,12
- Most Commonly Benign Fundic Gland Polyps (FGPs) 3
- 60% Sporadic but 40-80% Have > 100 Polyps 13
- Incidence: 21% 14
- Generally Benign with Low Risk of Distant Metastases 14
- Locally Aggressive with High Recurrence Rates 3,14
- Third Most Common Cause of Death in FAP 15
- Intraabdominal/Mesenteric Fibromatosis is the Most Common Subtype 16
- Incidence: 65-80% 3
- Most Common in the Frontal Bones 17
- Can Also Affect the Mandible, Maxilla, or Long Bones
- Range in Size from Slight Thickening to Large Palpable Masses 3
- 3x Overall Increased Risk 18
- Associated Tumors: 18
- Medulloblastoma – Most Common (13x Increased Risk)
- Astrocytoma
- Less Commonly: Ependymoma, Pinealblastoma, Ganglioglioma
- Most Commonly Develop During Childhood 18
- Previously Described as Turcot Syndrome – Now Only Considered a Part of the FAP Spectrum
Congenital Hypertrophy of Retinal Pigment Epithelium (CHRPE)
- Incidence: 90% 19
- The Most Common and Earliest Extraintestinal Manifestation of FAP 20,21
- Described as At Least One Dark Pigmented Lesion with a Halo in the Retina 22
- No Malignant Potential 23
Dental Abnormalities
- Incidence: 30-75% 25
- Include Impacted Teeth, Tooth Ankylosis, Missing Teeth, Supernumerary Teeth, Hypercementosis, or Compound Odontomas 25,26
Other Less Common Associations 3
- Thyroid Cancer
- Nasopharyngeal Angiofibroma
- Benign Skin Tumors
- Epidermal Cyst
- Fibroma
- Lipoma
- Pilomatricoma
- Hepatoblastoma
- Pancreatic Cancer
- Adrenal Tumors
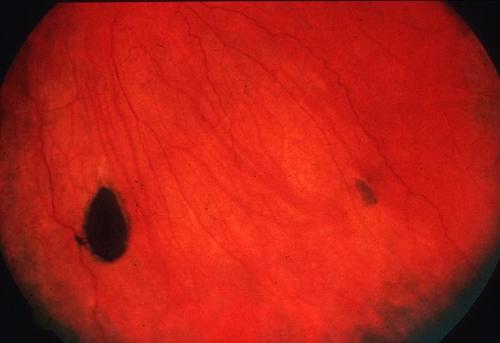
CHRPE 24
Variations
Classical FAP
- FAP as Described Above
Attenuated FAP (AFAP)
- Less Aggressive Phenotypic Variant
- Develop Fewer Polyps; Defined as < 100 Polyps (Oligopolyposis) 27
- Later Age of Diagnosis 28
- Later Age of Onset of Colorectal Cancer: Average Age 55 29
- Lower Risk of Colorectal Cancer (70-80%) 29,30
- Extracolonic Manifestations are Less Frequent 27
Gastric Adenocarcinoma and Proximal Polyposis of the Stomach (GAPPS)
- Defined as > 100 Polyps in the Proximal Stomach 27,31
- Polyps are Restricted to the Body and Fundus 31
- Spares the Antrum 31
- Predominantly Benign Fundic Gland Polyps (FGPs) 31
- Some Have Regions of Dysplasia with High Risk of Cancer
- No Evidence of Colorectal or Duodenal Polyps 27
Historical Variants
- Gardner’s Syndrome: FAP with Extracolonic Manifestations
- Turcot’s Syndrome: FAP with Brain Tumors
- *Originally Described Colonic Polyposis with Extracolonic Manifestations but Gardner’s Syndrome and Turcot’s Syndrome are Now Considered a Part of the FAP Spectrum 32
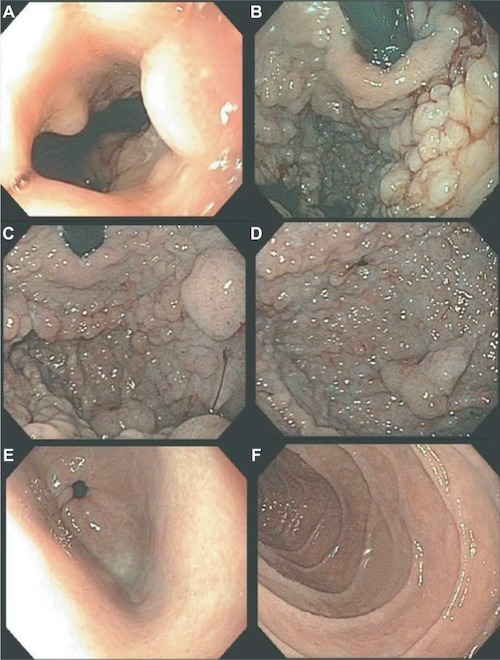
GAPPS on Upper Endoscopy: (A) GE Junction, (B) Cardia, (C) Fundus, (D) Body, (E) Spared Antrum, (F) Spared Duodenum 31
Diagnosis and Screening
Diagnosis
- Clinical Diagnosis Can Be Clear on Colonoscopy
- Flexible Sigmoidoscopy Alone is Often Sufficient
- Primary Diagnosis is Made by an APC Gene Mutation
- Patients with a Family History of FAP Should Undergo Genetic Counseling/Screening by Age 10-12 33
Colonoscopy
- High-Quality Colonoscopy Starting at Age 10-15 Years 34
- Repeat Every Year 34
- Flexible Sigmoidoscopy May Be Considered Based on patient and Family preference or Clinical Judgment 34
- Risk for Missing Transverse or Right-Sided Colon Polyps
Upper Endoscopy
- Start Screening at Age 20-25 34
- Start Immediately if Any Colon Polyps are Seen
- Repeat Endoscopy Based on Spigelman Stage 34
- Stage 0: Every 3-5 Years
- Stage I: Every 2-3 Years
- Stage II: Every 1-2 Years
- Stage III: Every 6-12 Months
- Stage IV: Expert Surveillance Every 3-6 Months
Other Screening Considerations
- May Also Consider Screening with Thyroid Ultrasound Every 2-5 Years Starting in Late Teenage Years 34
- No Specific Guidelines for Desmoid Tumor or Brain Tumor Surveillance
Surgical Management
Indications for Colectomy
- Absolute Indications (2015 ACG Guidelines): 35
- Documented or Suspected Colorectal Cancer
- Significant Symptoms
- Relative Indications (2015 ACG Guidelines): 35
- Multiple Large Adenomas > 6 mm
- Significant Increase in Adenoma Number on Consecutive Exams
- Adenoma with High-Grade Dysplasia
- Inability to Adequately Survey the Colon Because of Multiple Diminutive Polyps
- Consider Prophylactic Colectomy if Otherwise Not Indicated 36
- Exact Timing is Debated
- Most Patients Undergo Surgery Between Ages 15-25 Years 36
Surgical Options
- Total Abdominal Colectomy with Ileorectal Anastomosis (TAC/IRA)
- Spares Rectum
- Advantages:
- Technically Easier
- Lower Risk of Complications
- No Risk for Sexual or Bladder Dysfunction
- No Permanent Stoma
- Highest Risk of Metachronous Cancer in the Remaining Rectum (5-25% Future Risk)
- Generally Contraindicated for Severe Rectal Disease or if the Patient is Not Reliable for Follow-Up Surveillance
- Requires Flexible Proctoscopy Every 6-12 Months 34
- Total Proctocolectomy with Ileal Pouch Anal Anastomosis (IPAA)
- Resects the Majority of the Rectum – More Complex Operation
- Advantages:
- Lower Risk of Metachronous Cancer than TAC/IRA
- No Permanent Stoma
- Risk for Sexual or Bladder Dysfunction and Possible Fecal Incontinence
- Requires Flexible Pouchoscopy Every 6-12 Months 34
- Total Proctocolectomy with End Ileostomy
- Requires a Permanent Stoma
- Risk for Sexual or Bladder Dysfunction
- Requires Flexible Endoscopy of Ostomy Every Year 34
Other Colorectal Cancer and Polyposis Syndromes
Syndromes
- Familial Adenomatous Polyposis (FAP)
- Lynch Syndrome
- Juvenile Polyposis Syndrome (JPS)/Familial Juvenile Polyposis
- MUT Y Homolog (MUTYH)-Associated Polyposis (MAP)
- Peutz-Jeghers Syndrome (PJS)
- Serrated Polyposis Syndrome (SPS)
- PTEN Hamartoma Tumor Syndromes: (PHTS)
Comparisons
References
- Burt RW, Jasperson K. Familial Adenomatous Polyposis. National Organization for Rare Disorders (NORD). 2014.
- Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009 Oct 12;4:22.
- Dinarvand P, Davaro EP, Doan JV, Ising ME, Evans NR, Phillips NJ, Lai J, Guzman MA. Familial Adenomatous Polyposis Syndrome: An Update and Review of Extraintestinal Manifestations. Arch Pathol Lab Med. 2019 Nov;143(11):1382-1398.
- Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110(2):223.
- Ma H, Brosens LA, Offerhaus GJA, Giardiello FM, de Leng WW, Montgomery EA. Pathology and genetics of hereditary colorectal cancer. Pathology. 2018;50(1):49–59.
- Petersen GM, Slack J, Nakamura Y. Screening guidelines and premorbid diagnosis of familial adenomatous polyposis using linkage. Gastroenterology. 1991;100:1658–1664.
- Giardiello FM, Brensinger JD, Petersen GM. AGA technical review on hereditary colorectal cancer and genetic testing. Gastroenterology. 2001;121(1):198–213.
- Makarewicz W, Bobowicz M, Sawicka W, Rzyman W. The treatment of chronic pleural empyema with laparoscopic omentoplasty. Initial Report. Wideochir Inne Tech Maloinwazyjne. 2014 Dec;9(4):548-53. (License: CC BY-NC-ND 3.0)
- Brosens LA, Keller JJ, Offerhaus GJA, Goggins M, Giardiello FM. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005;54(7):1034–1043.
- Spigelman A, Talbot I, Williams C, Domizio P, Phillips R. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;334(8666):783–785.
- Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK: Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989, 2: 783-785. 10.1016/S0140-6736(89)90840-4.
- Abraham SC. Fundic gland polyps: common and occasionally problematic lesions. Gastroenterol Hepatol (N Y). 2010;6(1):48–51.
- Arnason T, Liang WY, Alfaro E, et al. Morphology and natural history of familial adenomatous polyposis-associated dysplastic fundic gland polyps. Histopathology. 2014;65(3):353–362.
- Slowik V, Attard T, Dai H, Shah R, Septer S. Desmoid tumors complicating Familial Adenomatous Polyposis: a meta-analysis mutation spectrum of affected individuals. BMC Gastroenterol. 2015 Jul 16;15:84.
- Righetti AEM, Jacomini C, Parra RS, Almeida ALNRd, Rocha JJR, Féres O. Familial adenomatous polyposis and desmoid tumors. Clinics. 2011;66(10):1839–1842.
- Huss S, Nehles J, Binot E, et al. β-catenin (CTNNB1) mutations and clinicopathological features of mesenteric desmoid-type fibromatosis. Histopathology. 2013;62(2):294–304.
- Kesharwani PN, Tiwari R, Ramaiah A, Jairaj A, Anand R, Tiwari H. Multiple odontoma in Gardner syndrome: unusual report. Int J Appl Dent Sci. 2018;4(3):284–286.
- Attard TM, Giglio P, Koppula S, Snyder C, Lynch HT. Brain tumors in individuals with familial adenomatous polyposis: a cancer registry experience and pooled case report analysis. Cancer. 2007 Feb 15;109(4):761-6.
- Wallis YL, Macdonald F, Hultén M, et al. Genotype-phenotype correlation between position of constitutional APC gene mutation and CHRPE expression in familial adenomatous polyposis. Hum Genet. 1994;94(5):543–548.
- Pang C, Keung JW, Tang NL, Fan DS, Lau JW, Lam DS. Congenital hypertrophy of the retinal pigment epithelium and APC mutations in two Chinese families with familial adenomatous polyposis. Eye. 2000;14(pt 1):18–22.
- Traboulsi EI, Apostolides J, Giardiello FM, et al. Pigmented ocular fundus lesions and APC mutations in familial adenomatous polyposis. Ophthalmic Genet. 1996;17(4):167–174.
- Tiret A, Parc C. Fundus lesions of adenomatous polyposis. Curr Opin Ophthalmol. 1999;10(3):168–172.
- Touriño R, Conde-Freire R, Rodríguez-Aves T, López-Valladares MJ, Otero-Cepeda JL, Capeans C. Value of the congenital hypertrophy of the retinal pigment epithelium in the diagnosis of familial adenomatous polyposis. Int Ophthalmol. 2004;25(2):101–112.
- Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009 Oct 12;4:22. (License: CC BY 2.0)
- Cankaya AB, Erdem MA, Isler SC, et al. Oral and maxillofacial considerations in Gardner’s syndrome. Int J Med Sci. 2012;9(2):137–141.
- Butler J, Healy C, Toner M, Flint S. Gardner syndrome—review and report of a case. Oral Oncol Extra. 2005;41(5):89–92.
- Roncucci L, Pedroni M, Mariani F. Attenuated adenomatous polyposis of the large bowel: Present and future. World J Gastroenterol. 2017 Jun 21;23(23):4135-4139.
- Hernegger GS, Moore HG, Guillem JG. Attenuated familial adenomatous polyposis: an evolving and poorly understood entity. Dis Colon Rectum. 2002;45:127–134; discussion 134-136.
- Yen T, Stanich PP, Axell L, Patel SG. APC-Associated Polyposis Conditions. 1998 Dec 18 [updated 2022 May 12]. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews
- Kanth P. Familial Adenomatus Polyposis Syndromes. BMJ Best Practices. 2023.
- Rudloff U. Gastric adenocarcinoma and proximal polyposis of the stomach: diagnosis and clinical perspectives. Clin Exp Gastroenterol. 2018 Dec 3;11:447-459. (License: CC BY-NC 3.0)
- Novelli M. The pathology of hereditary polyposis syndromes. Histopathology. 2015;66(1):78–87.
- Barnard J. Screening and surveillance recommendations for pediatric gastrointestinal polyposis syndromes. J Pediatr Gastroenterol Nutr. 2009 Apr;48 Suppl 2(Suppl 2):S75-8.
- Familial Adenomatous Polyposis. Genetic/Familial High-Risk Assessment: Colorectal. Version 2.2022. National Comprehensive Cancer Network (NCCN).
- Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW; American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223-62; quiz 263.
- Campos FG. Surgical treatment of familial adenomatous polyposis: dilemmas and current recommendations. World J Gastroenterol. 2014 Nov 28;20(44):16620-9.