Peritoneal Carcinomatosis
Peritoneal Carcinomatosis
Dimitri Alexander Petrov, MD
Table of Contents
Background
Definition: Malignancy of the Peritoneum Covered in Multiple Carcinomatous Masses (“Peritoneal Studding”)
The Most Common Malignant Process of the Peritoneal Cavity
Etiology
- Primary Peritoneal Carcinomatosis: Originates within the Peritoneum
- Mesothelioma is the Most Common Primary Cause
- Secondary Peritoneal Carcinomatosis: Originates from Another Source
Secondary Sources
- Stomach
- Small Bowel
- Colorectal
- Appendix
- Ovarian – The Most Common Source
- Pancreas
- Liver
- Biliary Tract
- Kidney
- Breast – The Most Common Extra-Abdominal Source
- Lung
Peritoneal Implants Most Often Locate at Sites of Relative Physiologic Fluid Stasis – Pelvic Peritoneal Reflections, Paracolic Gutters, Superior Sigmoid Mesocolon, Ileocolic Region, and Right Subdiaphragmatic Space
Pseudomyxoma Peritonei (PMP): Describes Diffuse Gelatinous Ascites with Mucinous Peritoneal Implants
- Most Common Source: Mucinous Cystadenoma of Appendix
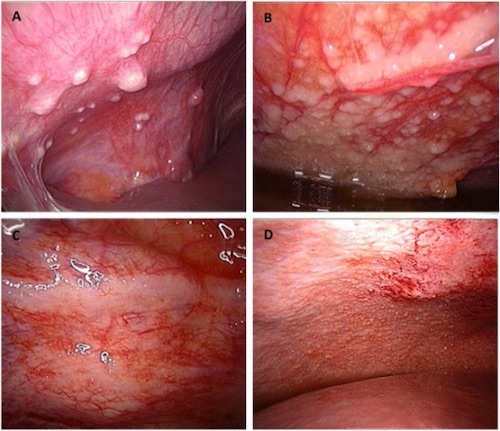
Peritoneal Carcinomatosis on Laparoscopy 1
Presentation and Diagnosis
Symptoms
- Increasing Abdominal Girth – The Most Common Presenting Symptom
- Ascites Caused by Increased Peritoneal Fluid Secretion and Impaired Reabsorption
- Inguinal Hernia – The Second Most Common Presenting Symptom
- Weight Loss
- Abdominal Pain
- Nausea and Vomiting
- Bowel Obstruction
- Diarrhea
- Dyspnea
Diagnosis Can Be Made by Imaging (CT), Paracentesis with Fluid Analysis, Omental Biopsy, or Laparoscopy with Implant Biopsy
Radiographic Findings
- Ascites
- “Organ Scalloping” – Scattered Peritoneal Nodules
- “Omental Caking” – Increased Density and Nodular Lesions
- Mesenteric Infiltration
- Stomach Wall Thickening
- Sleeve-Like Growth Along the Bowel Serosa
Paracentesis is Generally Able to Achieve Diagnose and Avoid the Need for More Invasive Laparoscopic Biopsy
Paracentesis Orders
- Cytology
- Cell Count and Differential
- Protein and Albumin
- Glucose
- Lactate Dehydrogenase
- Culture
If Discovered Intraoperatively for Small Bowel Obstruction (SBO): Biopsy Peritoneal Implants, Biopsy Omentum, Collect Fluid for Cytology, and Abort Operation
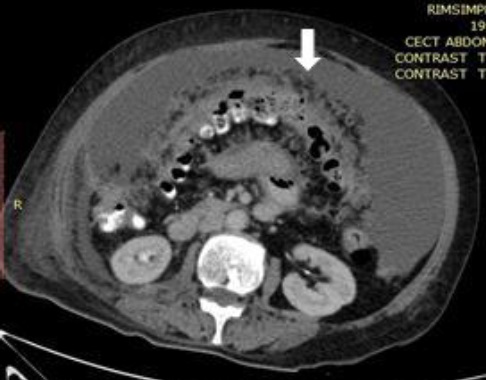
CT Showing Organ Scalloping (Arrow) of Peritoneal Carcinomatosis 2
Peritoneal Cancer Index (PCI)
Peritoneal Cancer Index (PCI)
- Grading Index Used for Research and to Objectively Describe the Extent of Tumor Burden
- Score = Sum of the Largest Lesion Size (0-3) in Each of 13 Defined Regions
- Scores Range from 0-39
Regions
- Region 0: Central
- Region 1: RUQ
- Region 2: Epigastrium
- Region 3: LUQ
- Region 4: Left Flank
- Region 5: LLQ
- Region 6: Pelvis
- Region 7: RLQ
- Region 8: Right Flank
- Region 9: Upper Jejunum
- Region 10: Lower Jejunum
- Region 11: Upper Ileum
- Region 12: Lower Ileum
Lesion Size Score
- 0 Points: No Tumor
- 1 Point: ≤ 0.5 cm
- 2 Points: ≤ 5.0 cm
- 3 Points: > 5.0 cm
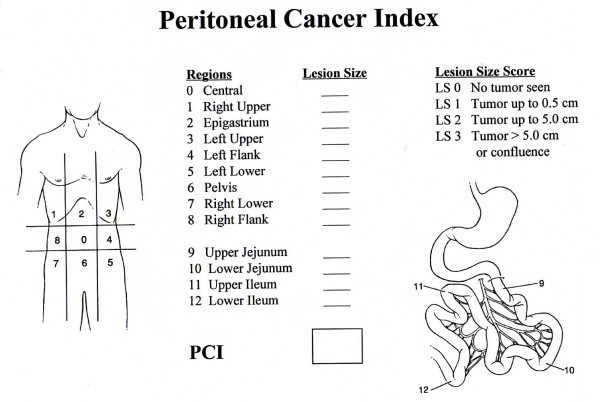
Peritoneal Cancer Index (PCI) Diagram 3
Management
Primary Treatment: Cytoreductive Surgery (CRS) and HIPEC
Managed Nonoperatively with Systemic Chemotherapy if Extraperitoneal Metastases or Other Contraindications are Present
Contraindications to CRS/HIPEC
- Absolute Contraindications:
- Extra-Abdominal Metastases
- Unresectable or Not Amenable to Complete Cytoreduction
- Multifocal Malignant Small Bowel Obstruction
- Poor Performance Status – Heart Failure, COPD, Renal Failure, etc.
- Relative Contraindications:
- Disease Progression on Systemic Therapy
- Short-Disease Free Interval (< 6 Months) – If Metachronous
- Peritoneal Cancer Index (PCI) > 20
- High-Grade Adenocarcinoma
- Elderly (Age ≥ 65 Years)
- Morbid Obesity (BMI ≥ 40)
Cytoreductive Surgery (CRS)
- Definition: Surgery to Decrease the Tumor Burden
- Resect All Bulky Tumor Implants and Leave No Residual Tumors > 2 mm
- Chemotherapy (HIPEC) is Unable to Reliably Penetrate Tumors > 2 mm – No Survival Benefit without a Complete Cytoreduction
- MNEMONIC: Nothing Over 2 mm to Move onto the 2nd Step
- Abdominal Wall Masses Should Be Excised with the Associated Peritoneum
Completeness of Cytoreduction (CC) Score
- A Scoring System Used to Grade How Complete Cytoreduction Was
- Scores:
- CC-0: No Disease
- CC-1: ≤ 2.5 mm
- CC-2: ≤ 2.5 cm
- CC-3: > 2.5 cm
- CC-0 and CC-1 are Considered “Complete Cytoreduction”
- CC-2 and CC-3 are Considered “Incomplete Cytoreduction”
- CC Score is a Significant Prognostic Indicator
HIPEC (Hyperthermic Intraperitoneal Chemotherapy)
- Heated Chemotherapeutic Drugs are Infused into the Peritoneal Cavity
- Avoids Systemic Circulation to Minimize Toxicity
- Heating Increases Penetration and Cytotoxicity
- Most Common Agents: Oxaliplatin, Cisplatin, Doxorubicin, and Mitomycin-C
- Performed After Surgical Debulking During the Same Operation – Adhesions Later Create a Barrier Preventing Uniform Distribution
- Requires No Residual Tumors > 2 mm for Chemotherapy to Penetrate
- Often Described as a “Shake and Bake” During an Approximately 30-120 Minute Infusion
EPIC (Early Postoperative Intraperitoneal Chemotherapy)
- Chemotherapy (Not Heated) is Given Via a Catheter or Subcutaneous Port
- Administered on Postoperative Days #1-5 After Surgical Debulking
- Able to Give Multiple Cycles with Longer Dwell Times
- Greater Risk of Systemic Absorption/Toxicity – HIPEC is Typically Favored
Management of Malignant Bowel Obstruction: *See Small Bowel Obstruction (SBO)
References
- Touboul C, Vidal F, Pasquier J, Lis R, Rafii A. Role of mesenchymal cells in the natural history of ovarian cancer: a review. J Transl Med. 2014 Oct 11;12:271. (License: CC BY-4.0)
- Singh S, Devi YS, Bhalothia S, Gunasekaran V. Peritoneal Carcinomatosis: Pictorial Review of Computed Tomography Findings. International Journal of Advanced Research. 2016;4(7):735–748. (License: CC BY-4.0)
- Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol. 2005 Feb 8;2(1):3. (License: CC BY-2.0)