Acute Respiratory Distress Syndrome (ARDS)
Johnathan W. Cain, DO
The Operative Review of Surgery. 2023; 1:29-40.
Table of Contents
General Information
Berlin Criteria 1 Mn
- Timing: Onset Must Be Within One Week of a Known Clinical Insult or New/Worsening Respiratory Symptoms
- Chest Imaging: Bilateral Opacities (Not Fully Explained by Effusions, Lobar Collapse, or Nodules)
- Origin: Respiratory Failure Cannot Be Fully Explained by Cardiac Failure or Fluid Overload
- Oxygenation: PaO2:FiO2 < 300
Severity 1
- *Based on PaO2/FiO2 (P:F Ratio)
- Mild: P:F ≤ 300
- Moderate: P:F ≤ 200
- Severe: P:F ≤ 100
Prevalence 3
- Mild: 30.0%
- Moderate: 46.6%
- Severe: 23.4%
Mortality
- Overall High Mortality (37-43%) 4,5
- Increases with Disease Severity 3,6
- Mild: 27-35%
- Moderate: 32-40%
- Severe: 45-46%
Causes 7-10
- Sepsis (31-43%) – Most Common Cause
- Pneumonia (40-42.3%)
- Aspiration (8-30%)
- Trauma (9-17%)
- Massive Transfusion/Transfusion-Related Acute Lung Injury (TRALI)
- Pancreatitis
- Inhalation Injury
- Cardiothoracic Surgery
- Medications
- Drugs
- Alcohol
- *Risk Increased by Simultaneous Factors 7
Clinical Features 11,12
- Dyspnea
- Tachypnea
- Tachycardia
- Altered Mental Status
- Respiratory Distress
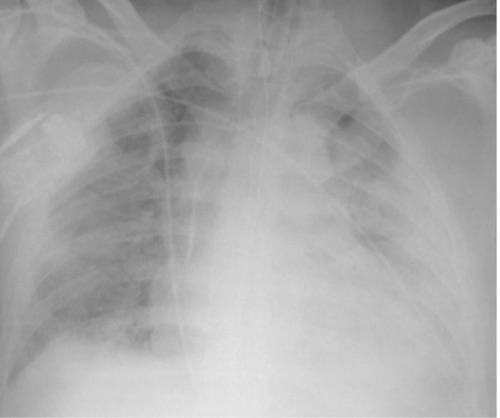
ARDS on Chest X-Ray 2
Phases and Pathophysiology
Starling Equation 13
- Describes Fluid Movement Between the Vasculature & Interstitium
- Q = K x [(Pc – Pi) – σ (πc – πi)]
- Q: Net Transvascular Flow
- K: Filtration Coefficient of Endothelial Membrane Permeability
- Pc: Capillary Hydrostatic Pressure
- Pi: Interstitial Hydrostatic Pressure
- σ: Reflection Coefficient of the Capillary Barrier
- πc: Capillary Oncotic Pressure
- πi: Interstitial Oncotic Pressure
- Normal Lung Function Prevents Alveolar Edema 14
- Retained Intravascular Proteins Increase Oncotic Pressure
- Interstitial Lymphatic Reabsorb Large Volumes of Fluid
- Tight Junctions Between Alveolar Epithelium Prevent Leakage
Stage 1 (Exudative Phase)
- Characterized by Diffuse Alveolar Damage (DAD) 15
- Occurs Over the First 7-10 Days
- Effects:
- Lung Injury Causes Release of Proinflammatory Cytokines (TNF, IL-1, IL-6, and IL-8) 16-19
- Causes Inflammation and Edema
- Cytokines Recruit Neutrophils Causing Release of Toxic Mediators that Further Damage Capillary/Alveolar Epithelium 20,21
- Necrosis and Sloughing of Type I Pneumocytes and Capillary Endothelium 22
- Loss of Tight Junctions that Normally Prevent Fluid Movement 22
- Increased Vascular Permeability Causes an Inflammatory Exudate (Protein Rich Fluid) to Flood the Alveoli 23
- Surfactant Inhibition Causes Collapse and Shunting 24
- Hyaline Membranes Form within the Alveoli 25
- Lung Injury Causes Release of Proinflammatory Cytokines (TNF, IL-1, IL-6, and IL-8) 16-19
Stage 2 (Fibroproliferative Phase)
- Characterized by Proliferation of Type II Pneumocytes 23
- Occurs After 7-10 Days
- Generally Lasts About 14-21 Days
- Effects:
- Early Collagen Formation 26,27
- Interstitial Infiltration of Myofibroblasts with Myointimal Thickening
- Squamous Metaplasia 28
- Decreased Compliance 29
- Effects are Still Reversible
Stage 3 (Fibrotic Phase)
- Characterized by Interstitial Fibrosis 30-32
- Not a Universal Outcome Seen in All Patients
- Associated with Prolonged Mechanical Ventilation and Increased Mortality 30,31
Complications
- Impaired Gas Exchange & Hypoxemia 33
- Primarily Due to a Ventilation-Perfusion Mismatch Due to Physiologic Shunting and Increased Dead Space 33
- The Most Common Long-Term Defect in Recovered Patients is Decreased Diffusion Capacity 34
- Decreased Lung Compliance 29
- Barotrauma 35
- Pulmonary Hypertension Due to Hypoxic Vasoconstriction, Vascular Compression Due to Positive Airway Pressure Ventilation, Airway Collapse, Parenchymal Destruction, and Hypercarbia 36
Diagnosis
Diagnosis 37
- Clinical Diagnosis Based on the Berlin Criteria 1
- Evaluation Should Focus on Identifying ARDS and the Underlying Cause
- Laboratory Testing is Nonspecific
- Imaging Includes CXR or CT
- Exclude Acute Cardiogenic Pulmonary Edema by Clinical Evaluation, BNP, and/or Echocardiogram 38,39
Berlin Criteria 1 Mn
- Timing: Onset Must Be Within One Week of a Known Clinical Insult or New/Worsening Respiratory Symptoms
- Chest Imaging: Bilateral Opacities (Not Fully Explained by Effusions, Lobar Collapse, or Nodules)
- Origin: Respiratory Failure Cannot Be Fully Explained by Cardiac Failure or Fluid Overload
- Oxygenation: PaO2:FiO2 < 300
Severity 1
- *Based on PaO2/FiO2 (P:F Ratio)
- Mild: P:F ≤ 300
- Moderate: P:F ≤ 200
- Severe: P:F ≤ 100
Lung Injury Prediction Score (LIPS) 40
- Scoring System to Evaluate the Risk for Developing ARDS
- Features: 40
- Predisposing Conditions:
- Shock (+2 Points)
- Aspiration (+2 Points)
- Sepsis (+1 Points)
- Pneumonia (+1.5 Points)
- High-Risk Surgery:
- Orthopedic Spine (+1 Points)
- Acute Abdomen (+2 Points)
- Cardiac (+2.5 Points)
- Aortic Vascular (+3.5 Points)
- Emergency Surgery (+1.5 Points)
- High-Risk Trauma:
- Traumatic Brain Injury (+2 Points)
- Smoke Inhalation (+2 Points)
- Near Drowning (+2 Points)
- Lung Contusion (+1.5 Points)
- Multiple Fractures (+1.5 Points)
- Risk Modifiers:
- Alcohol Abuse (+1 Points)
- Obesity (BMI > 30) (+1 Points)
- Hypoalbuminemia (+1 Points)
- Chemotherapy (+1 Points)
- FiO2 > 0.35 (> 4 L/min) (+2 Points)
- Tachypnea (RR > 30) (+1.5 Points)
- SpO2 < 95% (+1 Points)
- Acidosis (pH < 7.35) (+1.5 Points)
- Diabetes (Only if Sepsis) (-1 Points)
- Predisposing Conditions:
- Interpretation: 41
- Score ≤ 4: Low Risk of Developing ARDS
- Negative Predictive Value 97% – Better at Defining Patients at Low Risk than Defining Patients at High Risk 41
- Score > 4: High Risk of Developing ARDS
- Sensitivity 69%, Specificity 78% 41
- Score ≤ 4: Low Risk of Developing ARDS
Treatment
Primary Treatment
- Primarily Supportive Care 42
- Treat Underlying Pathology
- Nutritional Support 43
- General Critical Care Managements (VTE Prophylaxis, Hemodynamic Monitoring, Stress Ulcer Prophylaxis)
- Manage Patient-Ventilator Dyssynchrony
- Lung Protective Ventilation 44
- Conservative Fluid Management 45-47
- Goal CVP < 4 mmHg or PAOP < 8 mmHg 45
- May Require Diuretics as Long as the Patient is Hemodynamically Stable
- Liberal Fluid Management Has a Higher Risk for Pulmonary Edema
- Improves Ventilator-Free Days and ICU-Free Days 45
- No Clear Mortality Benefit 45
- Possibly an Increased Risk for Cognitive Impairment (Not Clear) 48
Lung Protective Ventilation
- Definition: Ventilation with Low Tidal Volumes to Reduce Alveolar Overdistention & Barotrauma
- Based Largely on ARDSnet Protocols 44
- Improves Mortality for ARDS of All Severities 49-53
- Ventilation (Tidal Volume/Respiratory Rate)
- Initial Tidal Volume: 8 ml/kg x Ideal Body Weight (General Standard) 44
- Decrease Tidal Volume 1 ml/kg Every 1-2 Hours 44
- Goal Tidal Volume: 4-6 ml/kg x Ideal Body Weight 44
- Initial Respiratory Rate Should Approximate Baseline Minute Ventilation (Not > 35 bpm) 44
- Adjust Tidal Volume & Respiratory Rate for Goal Plateau Pressure ≤ 30 cm H2O 44
- Oxygenation (PEEP/FiO2)
- Oxygenation Goals: PaO2 55-80 mmHg or SpO2 88-95% 44
- Adjust PEEP to Required FiO2
- Initially Recommended to Start with Lower-PEEP Strategy 44
| FiO2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 |
| PEEP | 5 | 5-8 | 8-10 | 10 | 10-14 | 14 | 14-18 | 18-24 |
- Consider High-PEEP Strategy if Refractory 44
| FiO2 | 0.3 | 0.4 | 0.5 | 0.6-0.7 | 0.8-0.9 | 1.0 |
| PEEP | 5-14 | 14-16 | 16-18 | 20 | 22 | 22-24 |
- Complications:
- Permissive Hypercapnia
- Respiratory Acidosis Allowed to Maintain Low Tidal Volumes
- Anticipated and Generally Well Tolerated 54,55
- pH Goal: 7.30-7.45 44
- If pH < 7.30: Increase Rate (Maximum 35 bpm) 44
- If pH Remains < 7.15: Can Increase Tidal Volume in 1 ml/kg Increments Until pH > 7.15 44
- PaCO2 Goal Not Well Defined
- Patient-Ventilator Dyssynchrony
- May Require Increased Sedation
- *See Patient-Ventilator Dyssynchrony
- Permissive Hypercapnia
Adjuncts
- Systemic Glucocorticoids (Steroids)
- Indicated for Moderate-Severe ARDS Refractory to Standard Treatments
- Generally Only Used if Early in the Disease Course (< 13-14 Days)
- May Also Use Steroids if Otherwise Indicated for Other Underlying Conditions
- Associated with Improved Mortality and Ventilator-Free Days 56-58
- Initiation 13-14 Days After Onset was Associated with Increased Mortality 59-61
- Dosing: Dexamethasone IV 20 mg Once Daily x5 Days, Then Reduce to 10 mg Daily for Another 5 Days – Based on DEXA-ARDS Study
- *Dexamethasone is a Pure Glucocorticoid (Anti-Inflammatory Effects) without Mineralocorticoid Side Effects (Sodium Retention/Volume Overload)
- Indicated for Moderate-Severe ARDS Refractory to Standard Treatments
- Inhaled Pulmonary Vasodilators (Nitric Oxide/Prostacyclin)
- Improves Oxygenation for Severe ARDS with Refractory Hypoxemia 62-65
- No Proven Morbidity or Mortality Benefit 66
- *See Ventilator Management
- Paralysis/Neuromuscular Blockade
- Generally Reserved Only for Severe ARDS with Refractory Hypoxemia
- Early Short-Term (48 Hour) Paralytics May Improve Mortality in Severe ARDS (Debated with Conflicting Results) 67,68
- Prone Positioning
- Indicated for Severe ARDS with Refractory Hypoxemia 69
- May Also Consider as a Bridge to ECMO
- Associated with Improved Mortality for Severe ARDS 69,70
- *See Ventilator Management
- Indicated for Severe ARDS with Refractory Hypoxemia 69
- Extracorporeal Membrane Oxygenation (ECMO)
- Considered for Acute Severe Pulmonary Failure that is Potentially Reversible & Unresponsive or Conventional Measures
- Associated with Improved Mortality for Severe ARDS 71
- *See Mechanical Circulatory Support
- Alternative Modes of Ventilation:
- Airway Pressure Release Ventilation (APRV)
- High-Frequency Oscillatory Ventilation (HFOV)
- *See Mechanical Ventilation: Settings & Modes
Mnemonics
Berlin Criteria for ARDS
- “ABC-3”
- Acute Onset (< 7 Days)
- Bilateral Opacities
- CHF Not Fully Explained
- P:F < 300
References
- The ARDS Definition Task Force*. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA. 2012;307(23):2526–2533.
- Santos LC, Abreu CF, Xerinda SM, Tavares M, Lucas R, Sarmento AC. Severe imported malaria in an intensive care unit: a review of 59 cases. Malar J. 2012 Mar 29;11:96. (License: CC BY-2.0)
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016 Feb 23;315(8):788-800.
- Sedhai YR, Yuan M, Ketcham SW, Co I, Claar DD, McSparron JI, Prescott HC, Sjoding MW. Validating Measures of Disease Severity in Acute Respiratory Distress Syndrome. Ann Am Thorac Soc. 2021 Jul;18(7):1211-1218.
- Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008 May;133(5):1120-7.
- Fanelli V, Vlachou A, Ghannadian S, Simonetti U, Slutsky AS, Zhang H. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis. 2013 Jun;5(3):326-34.
- Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982 Jul;144(1):124-30.
- Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995 Feb;151(2 Pt 1):293-301.
- Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU: comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med. 1998 Apr;157(4 Pt 1):1159-64.
- Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa S, Fernández RL, Kacmarek RM; ALIEN Network. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011 Dec;37(12):1932-41.
- Acute Respiratory Distress Syndrome. 2020. National Health Service (NHS). https://www.nhs.uk/conditions/acute-respiratory-distress-syndrome/
- Acute Respiratory Distress Syndrome. 2022. National Institute of Health (NIH). https://www.nhlbi.nih.gov/health/ards#:~:text=The%20first%20symptom%20of%20ARDS,can%20develop%20at%20any%20age.
- Starling EH. The production and absorption of lymph. In: Schafer EA. editor. Textbook of Physiology. Vol. 1. London: Pentland; (1898). p. 285–311.
- Matthay MA. Acute hypoxemic respiratory failure: Pulmonary edema and ARDS. In: Chest Medicine. Essentials of Pulmonary and Critical Care Medicine, 3rd ed, George RB, Light RW, Matthay MA, et al (Eds), Williams & Wilkins, Baltimore 1995. p.593.
- Cardinal-Fernández P, Lorente JA, Ballén-Barragán A, Matute-Bello G. Acute Respiratory Distress Syndrome and Diffuse Alveolar Damage. New Insights on a Complex Relationship. Ann Am Thorac Soc. 2017 Jun;14(6):844-850.
- Martin TR. Lung cytokines and ARDS: Roger S. Mitchell Lecture. Chest. 1999 Jul;116(1 Suppl):2S-8S.
- Miller EJ, Cohen AB, Matthay MA. Increased interleukin-8 concentrations in the pulmonary edema fluid of patients with acute respiratory distress syndrome from sepsis. Crit Care Med. 1996 Sep;24(9):1448-54.
- Donnelly SC, Strieter RM, Reid PT, Kunkel SL, Burdick MD, Armstrong I, Mackenzie A, Haslett C. The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med. 1996 Aug 1;125(3):191-6.
- Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990 Jun;85(6):1936-43.
- Windsor AC, Mullen PG, Fowler AA, Sugerman HJ. Role of the neutrophil in adult respiratory distress syndrome. Br J Surg. 1993 Jan;80(1):10-7.
- Chollet-Martin S, Gatecel C, Kermarrec N, Gougerot-Pocidalo MA, Payen DM. Alveolar neutrophil functions and cytokine levels in patients with the adult respiratory distress syndrome during nitric oxide inhalation. Am J Respir Crit Care Med. 1996 Mar;153(3):985-90.
- Michaels AJ. Management of post traumatic respiratory failure. Crit Care Clin. 2004 Jan;20(1):83-99, vi – vii.
- Walkey AJ, Summer R, Ho V, Alkana P. Acute respiratory distress syndrome: epidemiology and management approaches. Clin Epidemiol. 2012;4:159-69.
- Günther A, Ruppert C, Schmidt R, Markart P, Grimminger F, Walmrath D, Seeger W. Surfactant alteration and replacement in acute respiratory distress syndrome. Respir Res. 2001;2(6):353-64.
- Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147-63.
- Clark JG, Milberg JA, Steinberg KP, Hudson LD. Type III procollagen peptide in the adult respiratory distress syndrome. Association of increased peptide levels in bronchoalveolar lavage fluid with increased risk for death. Ann Intern Med. 1995 Jan 1;122(1):17-23.
- Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998 Nov;158(5 Pt 1):1432-41.
- Cheung, Y., Graziano, P., & Leslie, K. O. (2010). Acute Lung Injury. Practical Pulmonary Pathology: A Diagnostic Approach, 117-136.
- Russotto V, Bellani G, Foti G. Respiratory mechanics in patients with acute respiratory distress syndrome. Ann Transl Med. 2018 Oct;6(19):382.
- Marshall R, Bellingan G, Laurent G. The acute respiratory distress syndrome: fibrosis in the fast lane. Thorax. 1998 Oct;53(10):815-7.
- Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J. 2014 Jan;43(1):276-85.
- Zapol WM, Trelstad RL, Coffey JW, Tsai I, Salvador RA. Pulmonary fibrosis in severe acute respiratory failure. Am Rev Respir Dis. 1979 Apr;119(4):547-54.
- Donahoe M. Acute respiratory distress syndrome: A clinical review. Pulm Circ. 2011 Apr-Jun;1(2):192-211.
- Zhi H, Ji X, Zhao Z, Liang H, Zhong S, Luo Y, Zhong M, Zhan C, Gao Y, Deng X, Li S, Li J, Zhong N, Jiang M, Chen R. Risk factors for impaired pulmonary diffusion function in convalescent COVID-19 patients: A systematic review and meta-analysis. EClinicalMedicine. 2022 Jul;49:101473.
- Rajdev K, Spanel AJ, McMillan S, Lahan S, Boer B, Birge J, Thi M. Pulmonary Barotrauma in COVID-19 Patients With ARDS on Invasive and Non-Invasive Positive Pressure Ventilation. J Intensive Care Med. 2021 Sep;36(9):1013-1017.
- Morelli A, Teboul JL, Maggiore SM, Vieillard-Baron A, Rocco M, Conti G, De Gaetano A, Picchini U, Orecchioni A, Carbone I, Tritapepe L, Pietropaoli P, Westphal M. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit Care Med. 2006 Sep;34(9):2287-93.
- Fernando SM, Ferreyro BL, Urner M, Munshi L, Fan E. Diagnosis and management of acute respiratory distress syndrome. CMAJ. 2021 May 25;193(21):E761-E768.
- Mohammad NS, Nazli R, Zafar H, Fatima S. Effects of lipid based Multiple Micronutrients Supplement on the birth outcome of underweight pre-eclamptic women: A randomized clinical trial. Pak J Med Sci. 2022 Jan-Feb;38(1):219-226.
- Lazzeri C, Cianchi G, Bonizzoli M, Batacchi S, Peris A, Gensini GF. The potential role and limitations of echocardiography in acute respiratory distress syndrome. Ther Adv Respir Dis. 2016 Apr;10(2):136-48.
- Trillo-Alvarez C, Cartin-Ceba R, Kor DJ, Kojicic M, Kashyap R, Thakur S, Thakur L, Herasevich V, Malinchoc M, Gajic O. Acute lung injury prediction score: derivation and validation in a population-based sample. Eur Respir J. 2011 Mar;37(3):604-9.
- Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H 3rd, Hoth JJ, Mikkelsen ME, Gentile NT, Gong MN, Talmor D, Bajwa E, Watkins TR, Festic E, Yilmaz M, Iscimen R, Kaufman DA, Esper AM, Sadikot R, Douglas I, Sevransky J, Malinchoc M; U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS). Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011 Feb 15;183(4):462-70.
- ARDS Treatment and Recovery. American Lung Association. https://www.lung.org/lung-health-diseases/lung-disease-lookup/ards/ards-treatment-and-recovery
- Martindale RG, McClave SA, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G; American College of Critical Care Medicine; A.S.P.E.N. Board of Directors. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009 May;37(5):1757-61.
- ARDSnet Ventilator Protocol. NHLBI ARDS Network. 2008. http://www.ardsnet.org/tools.shtml
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006 Jun 15;354(24):2564-75.
- Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, Blackwood B, Fan E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017 Feb;43(2):155-170.
- Grissom CK, Hirshberg EL, Dickerson JB, Brown SM, Lanspa MJ, Liu KD, Schoenfeld D, Tidswell M, Hite RD, Rock P, Miller RR 3rd, Morris AH; National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network. Fluid management with a simplified conservative protocol for the acute respiratory distress syndrome*. Crit Care Med. 2015 Feb;43(2):288-95.
- Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012 Jun 15;185(12):1307-15.
- Acute Respiratory Distress Syndrome Network; Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1301-8.
- Qadir N, Bartz RR, Cooter ML, Hough CL, Lanspa MJ, Banner-Goodspeed VM, Chen JT, Giovanni S, Gomaa D, Sjoding MW, Hajizadeh N, Komisarow J, Duggal A, Khanna AK, Kashyap R, Khan A, Chang SY, Tonna JE, Anderson HL 3rd, Liebler JM, Mosier JM, Morris PE, Genthon A, Louh IK, Tidswell M, Stephens RS, Esper AM, Dries DJ, Martinez A, Schreyer KE, Bender W, Tiwari A, Guru PK, Hanna S, Gong MN, Park PK; Severe ARDS: Generating Evidence (SAGE) Study Investigators; Society of Critical Care Medicine’s Discovery Network. Variation in Early Management Practices in Moderate-to-Severe ARDS in the United States: The Severe ARDS: Generating Evidence Study. Chest. 2021 Oct;160(4):1304-1315.
- Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009 Oct 20;151(8):566-76.
- Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013 Feb 28;2013(2):CD003844.
- Fuller BM, Mohr NM, Miller CN, Deitchman AR, Levine BJ, Castagno N, Hassebroek EC, Dhedhi A, Scott-Wittenborn N, Grace E, Lehew C, Kollef MH. Mechanical Ventilation and ARDS in the ED: A Multicenter, Observational, Prospective, Cross-sectional Study. Chest. 2015 Aug;148(2):365-374.
- Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER. Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med. 2006 Jan;34(1):1-7.
- Gendreau S, Geri G, Pham T, Vieillard-Baron A, Mekontso Dessap A. The role of acute hypercapnia on mortality and short-term physiology in patients mechanically ventilated for ARDS: a systematic review and meta-analysis. Intensive Care Med. 2022 May;48(5):517-534.
- Lin P, Zhao Y, Li X, Jiang F, Liang Z. Decreased mortality in acute respiratory distress syndrome patients treated with corticosteroids: an updated meta-analysis of randomized clinical trials with trial sequential analysis. Crit Care. 2021 Mar 26;25(1):122.
- Chaudhuri, D., Sasaki, K., Karkar, A. et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med 47, 521–537 (2021).
- Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP; COALITION COVID-19 Brazil III Investigators. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020 Oct 6;324(13):1307-1316.
- Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, Tolley EA. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998 Jul 8;280(2):159-65.
- Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–84.
- Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: Meta-analysis. BMJ. 2008;336:1006–9.
- Rossaint R, Falke KJ, López F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993 Feb 11;328(6):399-405.
- Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K Jr, Kelly KM, Smith TC, Small RJ; Inhaled Nitric Oxide in ARDS Study Group. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004 Apr 7;291(13):1603-9.
- Dellinger RP, Zimmerman JL, Taylor RW, Straube RC, Hauser DL, Criner GJ, Davis K Jr, Hyers TM, Papadakos P. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998 Jan;26(1):15-23.
- Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007 Apr 14;334(7597):779.
- Adhikari NK, Dellinger RP, Lundin S, Payen D, Vallet B, Gerlach H, Park KJ, Mehta S, Slutsky AS, Friedrich JO. Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med. 2014 Feb;42(2):404-12.
- Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guérin C, Prat G, Morange S, Roch A; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010 Sep 16;363(12):1107-16.
- National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, Grissom CK, Gundel S, Hayden D, Hite RD, Hou PC, Hough CL, Iwashyna TJ, Khan A, Liu KD, Talmor D, Thompson BT, Ulysse CA, Yealy DM, Angus DC. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N Engl J Med. 2019 May 23;380(21):1997-2008.
- Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013 Jun 6;368(23):2159-68.
- Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, Pesenti A, Guérin C, Mancebo J, Curley MA, Fernandez R, Chan MC, Beuret P, Voggenreiter G, Sud M, Tognoni G, Gattinoni L. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. 2010 Apr;36(4):585-99.
- Combes A, Peek GJ, Hajage D, Hardy P, Abrams D, Schmidt M, Dechartres A, Elbourne D. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med. 2020 Nov;46(11):2048-2057.

