Hemorrhagic Shock & Trauma Resuscitation
David Ray Velez, MD
The Operative Review of Surgery. 2023; 1:1-10.
Table of Contents
Hemorrhagic Shock
Definition
- Inadequate Oxygen Delivery & Tissue Perfusion Due to Blood Loss
- *A Form of Hypovolemic Shock
Pathophysiology
- Cellular Level
- Oxygen Delivery Unable to Meet Oxygen Demand
- Aerobic Metabolism Converted to Anaerobic Metabolism 1
- Produces: Oxygen Radicals, Lactic Acid & Inorganic Phosphates 1
- Release of DAMPs Incite Systemic Inflammatory Response 2
- Predictable Hemostasis Fails & Cells Die
- Organ Level
- Hypovolemia & Resultant Vasoconstriction Cause End-Organ Hypoperfusion & Damage
- Hypoperfusion of the Brain & Myocardium Lead to Cerebral Anoxia & Fatal Arrhythmia within Minutes 3
Acute Traumatic Coagulopathy (ATC)
- Also Called Trauma-Induced Coagulopathy (TIC)
- Present in 24.4% of Trauma Patients 4
- Associated with High Mortality 5
- Mechanisms:
- Activated Protein C (APC)
- Anticoagulant – Inactivates Factors Va and VIIIa
- Increased Activity in Trauma 6,7
- Possibly Due to Upregulation of Thrombomodulin Activity in the Setting of Hypoperfusion
- Endothelial Glycocalyx Layer (EGL)
- “Shedding” of EGL After Injury Due to Yet Undetermined Mechanisms 8
- Anticoagulant Components Such as Chondroitin Sulfate and Heparan Sulfate 8
- Increased Fibrinolysis
- Clotting Cascade Activated Locally 8,9
- Distant Fibrinolytic Activity Increased 8,9
- Believed to Prevent Microvascular Thrombosis 8,9
- Platelet Impairment
- Numbers are Depleted 1
- Migration is Decreased 1
- Function is Impaired 1
- Activated Protein C (APC)
- The Lethal Triad (Hypothermia, Acidosis, & Coagulopathy) Compound on Each Other and Result in Significant Morbidity and Mortality 10
Hemorrhagic Shock Diagnosis
- First Step: Recognize its Presence 11
- May Miss if Only Looking at Blood Pressure Due to Early Compensation
- Earliest Signs: Tachycardia & Cutaneous Vasoconstriction 11
- Second Step: Determine the Cause 11
- Hemorrhagic Shock is Most Common in Trauma 11
- Obstructive Shock Caused by Cardiac Tamponade or Tension PTX 11
- Cardiogenic, Neurogenic or Septic Shock Can Also be Present
- Diagnosis Should Not Delay Appropriate Resuscitation
Possible Source of Occult Hemorrhage Mn
- Street/In the Field
- Chest
- Retroperitoneum
- Abdomen
- Pelvis
- Thighs
Hemorrhagic Shock Class Mn
| Class | Blood Loss | HR | BP | Pulse Pressure | RR | UOP | Mental Status |
| I | < 750 cc (< 15%) | Normal | Normal | Normal | Normal | Normal | Slightly Anxious |
| II | > 750 cc (15-30%) | > 100 | Normal | Narrow | > 20 | < 30 | Mildly Anxious |
| III | > 1500 cc (30-40%) | > 120 | Low | Narrow | > 30 | < 15 | Confused, Anxious |
| IV | > 2000 cc (> 40%) | > 140 | Low | Narrow | > 40 | 0 | Confused, Lethargic |
Damage Control Resuscitation
Initial Fluid Resuscitation
- Initial Step: 1-2 L Warmed Lactated Ringer Bolus 11
- *If Class III/IV Shock May Consider Immediate Transfusion to Blood/Blood Products to Limit Crystalloid Transfusions
- Pediatrics (If < 40 kg): 20 cc/kg 11
- Response: 11
- Rapid Responder
- Quick Correction that is Maintained
- Indicates Class I Shock
- Transient Responder
- Initially Responds but Then Deteriorates
- Indicates Class II-III Shock
- Non-Responder
- No Correction
- Indicates Class IV Shock
- Rapid Responder
- Next Step: 11
- Rapid Responder: No Further Immediate Boluses Required
- Transient or Non-Responders: Transition to Blood or Blood Products
- Strongly Consider Activation of Massive Transfusion Protocols (MTP) if Significant Volumes are Anticipated
Blood Transfusion
- Initial Blood: Type O pRBC
- Until Type & Crossmatch Available
- Approaches to Massive Transfusion Protocol (MTP)
- Hemostatic Resuscitation (1:1:1 Ratio)
- TEG-Guided Transfusion
- Whole Blood
Permissive Hypotension
- Also Known as: Hypotensive Resuscitation or Controlled Resuscitation
- Initial Goal SBP: ≥ 70 mmHg Until Definitive Hemostasis Achieved 12,13
- Rapid Resuscitation Exacerbates Bleeding By: 12,13
- Dislodging Fragile Clots
- Decreasing Blood Viscosity
- Exacerbating Lethal Triad
- Contraindicated in TBI – Maintenance of Cerebral Perfusion Pressure Essential to Prevent Secondary Brain Injury 14
- Age 15-49: ≥ 110 mmHg
- Age 50-69: ≥ 100 mmHg
- Age ≥ 70: ≥ 110 mmHg
Massive Transfusion Protocol (MTP)
Definitions
- ≥ 10 U pRBC in 24 Hours
- ≥ 4 U PRBC in 1 Hour
- *Generally Indicates a Large Volume of Blood/Blood Product Transfusion
Benefits 15
- Improves Survival
- Decreases Use of Blood Products
- Decreases Costs
Activation Indications
- Over a Dozen Scoring Systems 16
- Differ in Criteria & Significance
- Most Common Criteria: FAST, Hypotension, Tachycardia, Unstable Pelvic Fracture, & Low Hgb
- Scoring Systems:
- Trauma-Associated Severe Hemorrhage (TASH) Score – Most Well Validated if Using Exam, Labs, & FAST 16
- Assessment of Blood Consumptions (ABC) Score
- Vandromme Score
- Schreiber Score
- Shock Index
Hemostatic/Balanced Resuscitation
- pRBC:FFP:Plt at Ratio 1:1:1
- Closest Approximation to Whole Blood Available
- PROPPR RCT Demonstrated Decreased Death Due to Exsanguination 17
- Generally Consider the Standard Modern Approach
- Concentration After Dilution & Storage: 18,19
- Hematocrit: 29% (5-10% Are Lost After Transfusion)
- Platelet Count: 88,000 (Only 2/3 Are Viable After Transfusion)
- Coagulation Factors: 65% of Normal
- Effective Concentration (After Dilution, Storage & Immediate Losses): 18,19
- Hematocrit: 26%
- Platelet Count: 59,000
- Coagulation Factors: 65% of Normal
- *Adding More of One Component Dilutes the Other Two & Adding Fluids Dilutes All Three
- Barely Keeps Levels Above Traditional Transfusion Indications
Thrombelastography (TEG)
- Measures the Viscoelastic Properties of Clot Formation in Real Time
- Reading & Response:
- Allows More Rapid Goal-Directed Resuscitation than Conventional Coagulation Assays 20,21
- High-Grade Evidence of Improved Outcomes is Lacking
Whole Blood
- Better Access in Military with “Fresh Blood” from a “Walking Blood Bank” of Prescreened Soldiers 22,23
- Increasingly Becoming Available in Civilian Populations 24
- Concentration After Dilution & Storage: 19
- Hematocrit: 35-38%
- Platelet Count: 150,000-200,000
- Coagulation Factors: 85% of Normal
- May Decrease Transfusion Requirements & Mortality Although Evidence Insufficient
- *Many Still Advocate Whole Blood as the Best Available Resuscitation Product
Adjuncts
Tranexamic Acid (TXA)
- MOA: Inhibits Plasminogen Conversion to Plasmin, Inhibiting Fibrinolysis & Clot Breakdown
- Off-Label Use in the US
- Dosing: 1 g Bolus & Second 1 g Infusion Over 8 Hours
- Recommended for Significant Hemorrhage if Given Within 3 Hours of Injury 15
- *Based Largely on the CRASH-2 RCT 25
- If Given Within 3 Hours: Reduces Mortality and Blood Transfusions (Debated)
- If Given After 3 Hours: Increased Mortality 26
- No Evidence of Increased VTE Risk 15
Recombinant Activated Factor VIIa (rVIIa)
- MOA: Activates Factor X & Thrombin Formation
- Off-Label Use in the US
- Dosing: 200 μg/kg Bolus Followed By 100 μg/kg at One & Three Hours 27,28
- Generally Fallen Out of Favor
- *Initially There was Significant Hype but Subsequent Studies Fail to Demonstrate Any Improved Outcomes 15
Cryoprecipitate
- MOA: Replaces Fibrinogen
- Fibrinogen is the First Factor to Reach Critically Low Concentrations in Major Blood Loss & Low Levels are Associated with Poor Outcomes 29,30
- Insufficient Evidence to Guide Use
Vasopressors
- Historically Been Considered Heresy in Hemorrhagic Shock Due to Increased Mortality 31
- *Evidence is However Poor with High-Risk of Bias Through Observational Studies 32
- Arginine Vasopressin (AVP)
- Dosing: 4-U Bolus & 0.04-U/min Infusion
- AVERT Shock Trial: 33
- Decreased Transfusion Requirements
- No Change in Mortality
- No Increased Risk of Complications but May Decrease Risk of DVT
- Insufficient Evidence to Definitively Guide Use
Pneumatic Antishock Garment (PASG)/Military Antishock Trousers (MAST)
- Historical Tool, No Longer Used Today
- Inflatable Garment to Promote Hemostasis and Manually Increases Peripheral Vascular Resistance (PVR)
- 3 Inflatable Compartments: Abdomen/Pelvis and x2 Legs
- Each Inflated/Deflated Separately
- Was Previously Used in Pre-Hospital Settings
- Compartments Released One-At-A-Time Once in ED
- Contraindicated by Thoracic Trauma
- No Improvement in Mortality & May Even Worsen 34
- Can Cause Lower Extremity Ischemia/Compartment Syndrome 35
- Non-Pneumatic Anti-Shock Garment (NASG) – A Variation that Creates Compression by Tension without the Use of Inflation

MAST/PSAG 36
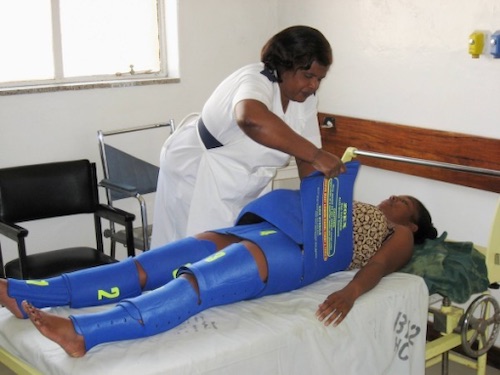
NASG 37
Mnemonics
Possible Source of Occult Hemorrhage
- Bloodied Patients Have Been “SCRAPT”
- S: Street/In the Field
- C: Chest
- R: Retroperitoneum
- A: Abdomen
- P: Pelvis
- T: Thighs
Hemorrhagic Shock Class (Percent Blood Loss)
- Tennis Scoring System – Similar to How the Game Tennis is Scored
- 0-15-30-40
- Class I: < 15%
- Class II: > 15%
- Class III: > 30%
- Class IV: > 40%
References
- Cannon JW. Hemorrhagic Shock. N Engl J Med. 2018 Jan;378(4):370-379.
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating Mitochondrial DAMPs Cause Inflammatory Responses to Injury. Nature. 2010 Mar;464(7285):104-107.
- Tisherman SA, Alam HB, Rhee PM, Scalea TM, Drabek T, Forsythe RM, Kochanek PM. Development of the Emergency Preservation and Resuscitation for Cardiac Arrest from Trauma Clinical Trial. J Trauma Acute Care Surg. 2017 Nov;83(5):803-809.
- Brohi K, Singh J, Heron M, Coats T. Acute Traumatic Coagulopathy. J Trauma. 2003;54:1127-1130.
- MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early Coagulopathy Predicts Mortality in Trauma. J Trauma. 2003;55:39-44.
- Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) Study: Comparative Effectiveness of a Time-Varying Treatment with Competing Risks. JAMA Surg. 2013 Feb;148(2):127-136.
- Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, Schreiber MA, Bulger EM, Muskat P, Alarcon LH, et al. Clinical and Mechanistic Drivers of Acute Traumatic Coagulopathy. J Trauma Acute Care Surg. 2013 Jul;75(1 Suppl 1):S40-47.
- Chang R, Cardenas JC, Wade CE, Holcomb JB. Advances in the Understanding of Trauma-Induced Coagulopathy. Blood. 2016 Aug;128(8):1043-1049.
- Hoffman M, Cichon LJH. Practical Coagulation for the Blood Banker. Transfusion. 2013 Jul;53(7):1594-1602.
- Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting Life-Threatening Coagulopathy in the Massively Transfused Trauma Patient: Hypothermia and Acidosis Revisited. J Trauma. 1997 May;42(5):857-861.
- Advanced Trauma Life Support (ATLS) Student Course Manual, 10th Am Coll Surg. 2018.
- Dutton RP, Mackenzie CF, Scalea TM. Hypotensive Resuscitation During Active Hemorrhage: Impact on In-Hospital Mortality. J Trauma. 2002 Jun;52(6):1141-1146.
- Tran A, Yates J, Lau A, Lampron J, Matar M. Permissive Hypotension Versus Conventional Resuscitation Strategies in Adult Trauma Patients with Hemorrhagic Shock: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Trauma Acute Care Surg. 2018 May;84(5):802-808.
- Carney N, Totten AM, O’Reilly C, Ulman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017 Jan;80(1):6-15.
- Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, Dubose JJ, Fox EE, Inaba K, Rodriguez CJ, et al. Damage Control Resuscitation in Patients with Severe Traumatic Hemorrhage: A Practice Management Guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017 Mar;82(3):605-617.
- Shih AW, Khan SA, Wang AY, Dawe P. Young PY, Greene A, Hudoba M, Vu E. Systematic Reviews of Scores and Predictors to Trigger Activation of Massive Transfusion Protocols. J Trauma Acute Care Surg. 2019 Sep;87(3):717-729.
- Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA. Transfusion of Plasma, Platelets and Red Blood Cells in a 1:1:1 vs a 1:1:2 Ratio and Mortality in Patients with Severe Trauma: The PROPPR Randomized Clinical Trial. JAMA. 2015 Feb;313(5):471-482.
- Mays JA, Hess JR. Modelling the Effects of Blood Component Storage Lesions on the Quality of Hemostatic Resuscitation in Massive Transfusion for Trauma. Blood Transfus. 2017 Mar;15(2):153-157.
- Cap AP, Beckett A, Benov A, Borgman M, Chen J, Corley JB, Doughty H, Fisher A, Glassberg E, Gonzales R, et al. Whole Blood Transfusion. Mil Med. 2018 Sep;183(Suppl 2):44-51.
- Unruh M, Reyes J, Helmer SD, Haan JM. An Evaluation of Blood Product Utilization Rates with Massive Transfusion Protocol: Before and After Thromboelastography (TEG) Use in Trauma. Am J Surg. 2019 Dec;218(6):1175-1180.
- Mohamed M, Majeske K, Sachwani GR, Kennedy K, Salib M, McCann M. The Impact of Early Thromboelastography Directed Therapy in Trauma Resuscitation. Scand J Trauma Resusc Emerg Med. 2017 Oct;15(1):99.
- Repine TB, Perkins JG, Kauvar DS, Blackborne L. The Use of Fresh Whole Blood in Massive Transfusion. J Trauma. 2006 Jun;60(6 Suppl):S59-S69.
- Vanderspurt CK, Spinella PC, Cap AP, Hill R, Matthews SA, Corley JB, Gurney JM. The Use of Whole Blood in US Military Operations in Iraq, Syria, and Afghanistan Since the Introduction of Low-Titer Type O Whole Blood: Feasibility, Acceptability, Challenges.
- Pivalizza EG, Stephens CT, Sridhar S, Gumbert SD, Rossmann S, Bertholf MF, Bai Y, Cotton BA. Whole Blood for Resuscitation in Adult Civilian Trauma in 2017: A Narrative Review. Anesth Analg. 2018 Jul;127(1):157-162.
- CRASH-2 Trial Collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, et al. Effects of Tranexamic Acid on Death, Vascular Occlusive Events, and Blood Transfusion in Trauma Patients with Significant Haemorrhage (CRASH-2): A Randomised, Placebo-Controlled Trial. Lancet. 2010 Jul;376(9734):23–32.
- CRASH-2 Trial Collaborators, Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, et al. The Importance of Early Treatment with Tranexamic Acid in Bleeding Trauma Patients: An Exploratory Analysis of the CRASH-2 Randomized Control Trial. Lancer. 2011 Mar;377(9771):1096-1101.
- Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, Leppaniemi A, Parr M, Vincent JL, Tortella BJ, et al. Results of the CONTROL Trial: Efficacy and Safety of Recombinant Activated Factor VII in the Management of Refractory Traumatic Hemorrhage. J Trauma. 2010 Sep;69(3):489–500.
- Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, Axelsen M, Kluger Y, NovoSeven Trauma Study Group. Recombinant Factor VIIa as Adjunctive Therapy for Bleeding Control in Severely Injured Trauma Patients: Two Parallel Randomized, Placebo-Controlled, Double-Blind Clinical Trials. J Trauma. 2005 Jul;59(1):8–15.
- Hiippala ST, Myllyla GJ, Vahtera EM. Hemostatic Factors and Replacement of Major Blood Loss with Plasma-Poor Red Cell Concentrates. Anesth Analg. 1995 Aug;81(2):360-365.
- Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K. Fibrinogen Levels During Trauma Hemorrhage, Response to Replacement Therapy, and Association with Patient Outcomes. J Thromb Haemost. 2012 Jul;10(7):1342-1351.
- Sperry JL, Minei JP, Frankel HL, West MA,Harbrecht BG, Moore EE, Maier RV, Nirula R. Early Use of Vasopressors After Injury: Caution Before Constriction. J Trauma. 2008 Jan;64(1):9-14.
- Hylands M, Toma A, Beaudoin N, Frenette AJ, D’Aragon F, Belley-Cote E, Charbonney E, Moller MH, Lakke JH, Vandvik PO, et al. Early Vasopressor Use Following Traumatic Injury: A Systematic Review. BMJ Open. 2017 Nov;7(11):e017559.
- Sims CA, Holena D, Kim P, Pascual J, Smith B, Martin N, Seamon M, Shiroff A, Raza S, Kaplan L, et al. Effects of Low-Dose Supplementation of Arginine Vasopressin on Need for Blood Product Transfusion in Patients with Trauma and Hemorrhagic Shock. JAMA Surg. 2019 Nov;154(11):994-1003.
- Bickell WH, Pepe PE, Bailey ML, Wyatt CH, Mattox KL. Randomized trial of pneumatic antishock garments in the prehospital management of penetrating abdominal injuries. Ann Emerg Med. 1987 Jun;16(6):653-8.
- Christensen KS. Pneumatic antishock garments (PASG): do they precipitate lower-extremity compartment syndromes? J Trauma. 1986 Dec;26(12):1102-5.
- Lateef F, Kelvin T. Military anti-shock garment: Historical relic or a device with unrealized potential? J Emerg Trauma Shock. 2008 Jul;1(2):63-9. (License: CC BY 2.0)
- Miller S, Fathalla MM, Ojengbede OA, Camlin C, Mourad-Youssif M, Morhason-Bello IO, Galadanci H, Nsima D, Butrick E, Al Hussaini T, Turan J, Meyer C, Martin H, Mohammed AI. Obstetric hemorrhage and shock management: using the low technology Non-pneumatic Anti-Shock Garment in Nigerian and Egyptian tertiary care facilities. BMC Pregnancy Childbirth. 2010 Oct 18;10:64. (License: CC BY 2.0)

