Vasoactive Intestinal Peptide-Secreting Tumor (VIPoma)
Forest Washington, MD
The Operative Review of Surgery. 2023; 1:182-188.
Table of Contents
Pathophysiology
Also Known as “VIP-Producing Neuroendocrine Tumor”, “Vasoactive Intestinal Peptide-Secreting Neoplasm”, or “VIP-Secreting Tumor of the Pancreas”
Definition 1
- Vasoactive Intestinal Peptide (VIP) Secreting Neuroendocrine Tumor
- Most Commonly Arise from Islet Cells of the Pancreas (75-90%) 2
- *See Pancreatic Neuroendocrine Tumor (PNET)
- Other Potential Sites: 2-5
- Lung
- Colon
- Liver
- Adrenal Gland or Sympathetic Ganglion – More Common in Infants and Pediatric Patients
- Neuroblastoma
- Ganglioneuroma
- Pheochromocytoma
- Paraganglioma
Distribution and Size
- Most Common in Body or Tail of the Pancreas (70-75%) 6-8
- Usually Solitary with Rare Multicentric Masses (5%) 9,10
- Most are Large (> 3 cm) 6,11
Malignancy
- Most are Malignant and Have Metastasized at the Time of Diagnosis (60-80%) 9
- 5% Have Multiple Endocrine Neoplasia Type 1 (MEN-1) 12
Epidemiology
- Median Age: 40-50 Years 2,13,14
- Between Ages 2-4 Years in Children 4
- More Common in Females (65%) 14
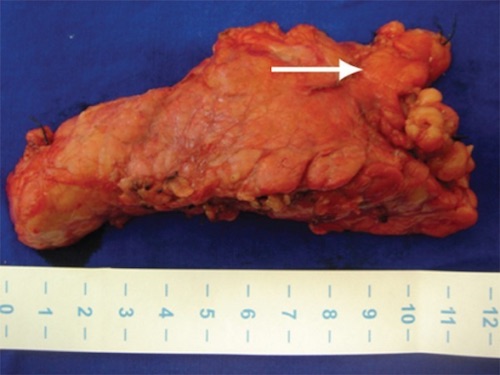
Distal Pancreatectomy Specimen with a Peripancreatic VIPoma (Arrow) 15
Presentation
VIPoma Syndrome
- Also Known As:
- Watery Diarrhea, Hypokalemia, and Achlorhydria (WDHA) Syndrome
- Verner-Morrison Syndrome
- Pancreatic Cholera
- Classic Triad: 2,4
- Watery Diarrhea
- Profuse and Persists with Fasting (> 700 mL/Day but Can Be Over 6 L)
- Tea-Colored Odorless Stools
- Profound Diarrhea Can Lead to Electrolyte Imbalances and Non-Anion Gap Metabolic Acidosis
- Hypokalemia
- Due to Diarrhea, Aldosterone Synthesis, and Direct Excretion by Enterocytes
- Achlorhydria (HCl)
- Due to the Inhibition of HCl Secretion by Gastric Parietal Cells
- Watery Diarrhea
Additional Symptoms 2,4,16-18
- Flushing (15-30%)
- Hyperglycemia (20-50%)
- Hypercalcemia (25-50%)
- Nausea and Vomiting
- Lethargy
- Weakness
- Muscle Cramps
- Weight Loss
Diagnosis
Diagnosis
- Primary Diagnosis Made by Demonstrating High Serum VIP Levels (> 200 pg/mL) 19,20
- Biopsy Not Required but Can Be Used for Grading 21
TNM Staging
- Same System Used for all Pancreatic Neuroendocrine Tumors 22
- *See Pancreatic Neuroendocrine Tumor (PNET)
Localization
- Initial Imaging: Noninvasive (CT or MRI) 23,24
- Somatostatin Receptor Imaging 23,24
- Consider if Initial Imaging Fails to Localize
- Options:
- Somatostatin (Octreotide) Receptor Scintigraphy (SRS) – Classic Test Used
- Functional PET Scan (Ga-68 DOTATATE) – Becoming More Prevalent with Higher Sensitivity
- If Noninvasive Imaging Fails: Invasive Imaging
- Endoscopic Ultrasound (EUS) – Generally Preferred Next Step 25
- Selective Visceral Angiography
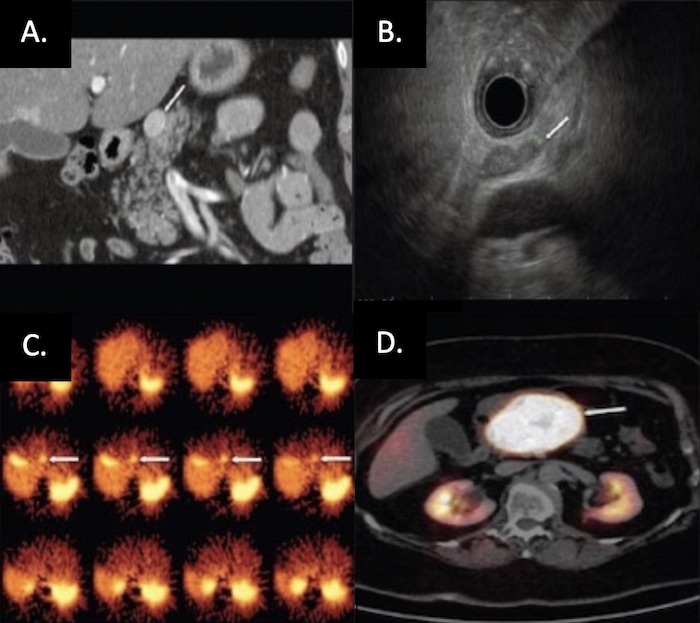
PNET on Imaging: (A) CT, (B) EUS, (C) SRS, (D) Functional PET 26
Treatment
Initial Medical Therapy 27-29
- Aggressive Fluid Resuscitation
- Electrolyte Replacement
- Somatostatin Analogs (Octreotide/Lanreotide) May Be Needed to Control Diarrhea
- Additional Agents for Refractory Diarrhea:
- Glucocorticoids
- Clonidine
- Loperamide
Surgical Resection (Treatment of Choice) 2,30,31
- Definitive Treatment: Surgical Resection
- Head/Neck: Pancreaticoduodenectomy
- Body/Tail: Distal Pancreatectomy (Concurrent Splenectomy if Malignancy is Suspected)
- Entire Pancreas: Total Pancreatectomy
- May Consider Enucleation for Small Tumors (< 2-3 cm) – Controversial Due to High Malignancy Rates
- Additional Requirements: Single Lesion, ≥ 2-3 mm From the Main Pancreatic Duct, Well-Encapsulated, and No Local Invasion
- Due to High Rates of Metastases, Surgical Resection is Often Not Feasible 9
Liver-Directed Therapy
- Resection of Metastases if Able
- Radiofrequency Ablation (RFA) or Cryoablation 32
- Hepatic Artery Embolization 33,34
Additional Options in Surgically Unresectable Disease
- Somatostatin Analogs (Octreotide/Lanreotide) to Decrease Hormonal Secretion and Control Symptoms 28,29
- Other Molecular-Targeted Agents:
- Everolimus 35
- Sunitinib 36
- Peptide Receptor Radionuclide Therapy (PRRT) 37,38
- Chemotherapy 39,40
- Radiation Therapy 41,42
- Pancreatic Neuroendocrine Carcinomas Were Previously Considered to be Resistant to Radiation
References
- National Cancer Institute. NIH.
- de Herder WW, Hofland J. Vasoactive Intestinal Peptide-Secreting Tumor (VIPoma). 2023 Apr 5. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext.
- Krejs GJ: VIPoma syndrome. Am J Med. 82:37–48. 1987.
- Abdullayeva, L.”VIPoma: Mechanisms, clinical presentation, diagnosis and treatment (Review)”. World Academy of Sciences Journal 1.5 (2019): 229-235.
- Ghaferi AA, Chojnacki KA, Long WD, Cameron JL and Yeo CJ: Pancreatic VIPomas: Subject review and one institutional experience. J Gastrointest Surg. 12:382–393. 2008.
- Soga J, Yakuwa Y. Vipoma/diarrheogenic syndrome: a statistical evaluation of 241 reported cases. J Exp Clin Cancer Res. 1998;17(4):389–400.
- Siddappa PK, Vege SS. Vasoactive Intestinal Peptide-Secreting Tumors: A Review. 2019;48(9):1119–25.
- Smith SL, Branton SA, Avino AJ, Martin JK, Klingler PJ, Thompson GB, et al. Vasoactive intestinal polypeptide secreting islet cell tumors: a 15-year experience and review of the literature. 1998;124(6):1050–5.
- Una Cidon E. Vasoactive intestinal peptide secreting tumour: An overview. World J Gastrointest Oncol. 2022 Apr 15;14(4):808-819.
- Parbhu SK, Adler DG. Pancreatic neuroendocrine tumors: contemporary diagnosis and management. Hosp Pract (1995) 2016;44:109–119.
- Batcher E, Madaj P, Gianoukakis AG. Pancreatic neuroendocrine tumors. Endocr Res. 2011;36:35–43.
- Parbhu SK and Adler DG: Pancreatic neuroendocrine tumors: Contemporary diagnosis and management. Hosp Pract 1995. 44:109–119. 2016.
- Mete O, Basturk O, Brosens LA, de Herder W, Hong SM, La Rosa S. VIPoma. In: Klimstra DS, Osamura RY, editors. WHO Classification of Tumours Endocrine and neuroendocrine tumours. Lyon: International Agency for Research on Cancer; 2023.
- Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A, Hassan M, Evans DB. Population based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492–3500.
- Apodaca-Torrez FR, Triviño M, Lobo EJ, Goldenberg A, Triviño T. Extra-pancreatic vipoma. Arq Bras Cir Dig. 2014 Jul-Sep;27(3):222-3. (License: CC BY-NC 4.0)
- Piet R, Dunckley H, Lee K and Herbison AE: Vasoactive intestinal peptide excites GnRH neurons in male and female mice. Endocrinology. 157:3621–3630. 2016.
- Brentjens R and Saltz L: Islet cell tumors of the pancreas: The medical oncologist’s perspective. Surg Clin North Am. 81:527–542. 2001.
- Schizas D, Mastoraki A, Bagias G, Patras R, Moris D, Lazaridis II, Arkadopoulos N and Felekouras E: Clinicopathological data and treatment modalities for pancreatic vipomas: A systematic review. J BUON. 24:415–423. 2019.
- Bloom SR. Vasoactive intestinal peptide, the major mediator of the WDHA (pancreatic cholera) syndrome: value of measurement in diagnosis and treatment. Am J Dig Dis. 1978;23(4):373–6.
- Schizas D, Mastoraki A, Bagias G, Patras R, Moris D, Lazaridis II, et al. Clinicopathological data and treatment modalities for pancreatic vipomas: a systematic review. J buon. 2019;24(2):415–23.
- Perren A, Couvelard A, Scoazec JY, Costa F, Borbath I, Delle Fave G, Gorbounova V, Gross D, Grossma A, Jense RT, Kulke M, Oeberg K, Rindi G, Sorbye H, Welin S. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. 2017;105(3):196–200.
- Bergsland EK, Woltering EA, Rindo G. Neuroendocrine tumors of the pancreas. In: AJCC Cancer Staging Manual, 8th ed, Amin MB (Ed), AJCC, Chicago 2017. p.407. Corrected at 4th printing, 2018.
- Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020 Jul;31(7):844-860.
- Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103(2):153-71.
- Rösch T, Lightdale CJ, Botet JF, Boyce GA, Sivak MV Jr, Yasuda K, Heyder N, Palazzo L, Dancygier H, Schusdziarra V, et al. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med. 1992 Jun 25;326(26):1721-6.
- Kartalis N, Mucelli RM, Sundin A. Recent developments in imaging of pancreatic neuroendocrine tumors. Ann Gastroenterol. 2015 Apr-Jun;28(2):193-202. (License: CC BY-NC-SA 3.0)
- Dimitriadis GK, Weickert MO, Randeva HS, Kaltsas G, Grossman A. Medical management of secretory syndromes related to gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2016 Sep;23(9):R423-36.
- O’Dorisio TM, Mekhjian HS, Gaginella TS. Medical therapy of VIPomas. Endocrinol Metab Clin North Am. 1989 Jun;18(2):545-56.
- Kaltsas G, Caplin M, Davies P, Ferone D, Garcia-Carbonero R, Grozinsky-Glasberg S, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pre- and Perioperative Therapy in Patients with Neuroendocrine Tumors. 2017;105(3):245–54.
- Lapeña Rodríguez M, Cholvi Calduch R, Muñoz Forner E, Garcés Albir M and Sabater Ortí L: Life-threating diarrhea and acute renal failure secondary to pancreatic VIPoma treated by surgery. Rev Esp Enferm Dig. 111:641–643. 2019.
- Partelli S, Bartsch DK, Capdevila J, Chen J, Knigge U, Niederle B, Nieveen van Dijkum EJM, Pape UF, Pascher A, Ramage J, et al: Antibes consensus conference participants: ENETS consensus guidelines for standard of care in neuroendocrine tumours: Surgery for small intestinal and pancreatic neuroendocrine tumours. Neuroendocrinology. 105:255–265. 2017.
- Moug SJ, Leen E, Horgan PG, Imrie CW. Radiofrequency ablation has a valuable therapeutic role in metastatic VIPoma. Pancreatology. 2006;6(1-2):155-9.
- Gupta S, Johnson MM, Murthy R, Ahrar K, Wallace MJ, Madoff DC, McRae SE, Hicks ME, Rao S, Vauthey JN, Ajani JA, Yao JC. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005 Oct 15;104(8):1590-602.
- Arrese D, McNally ME, Chokshi R, Feria-Arias E, Schmidt C, Klemanski D, Gregory G, Khabiri H, Shah M, Bloomston M. Extrahepatic disease should not preclude transarterial chemoembolization for metastatic neuroendocrine carcinoma. Ann Surg Oncol. 2013 Apr;20(4):1114-20.
- Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011 Feb 10;364(6):514-23.
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011 Feb 10;364(6):501-13.
- Zandee WT, Brabander T, Blazevic A, Kam BLR, Teunissen JJM, Feelders RA, et al. Symptomatic and Radiological Response to 177Lu-DOTATATE for the Treatment of Functioning Pancreatic Neuroendocrine Tumors. J Clin Endocrinol Metab. 2019;104(4):1336–44.
- Audil HY, Eiring RA, Kendi AT, Halfdanarson TR. A Case of Metastatic VIPoma With Complete Response to Peptide Radionuclide Receptor Therapy. 2021;50(4):e45–e6.
- Marques B, Monteiro AR, Martins RG, Couto J, Rodrigues F, Ribeiro J. Metastatic VIPoma, Cosecreting Insulin, With Complete Response to Lanreotide, Capecitabine, and Temozolomide. 2020;49(3):e19–e20.
- Brugel M, Walter T, Goichot B, Smith D, Lepage C, Do Cao C, et al. Efficacy of treatments for VIPoma: A GTE multicentric series. 2021;21(8):1531–9.
- Contessa JN, Griffith KA, Wolff E, Ensminger W, Zalupski M, Lawrence TS, Ben-Josef E. Radiotherapy for pancreatic neuroendocrine tumors. Int J Radiat Oncol Biol Phys. 2009 Nov 15;75(4):1196-200.
- Strosberg J, Hoffe S, Gardner N, Choi J, Kvols L. Effective treatment of locally advanced endocrine tumors of the pancreas with chemoradiotherapy. Neuroendocrinology. 2007;85(4):216-20.

