Pancreatic Neuroendocrine Tumor (PNET)
Henry William Taylor, MD, Forest Washington, MD, and Michael D. Smith, MD
The Operative Review of Surgery. 2023; 1:189-196.
Table of Contents
Definitions and Tumors
Definitions
- Pancreatic Neuroendocrine Neoplasia (PNEN) – Any Neuroendocrine Neoplasia of the Pancreas
- Previously Known as “Islet Cell Tumors”
- Pancreatic Neuroendocrine Tumor (PNET) – Well-Differentiated PNEN
- Pancreatic Neuroendocrine Carcinoma (PNEC) – Poorly-Differentiated PNEN with High-Proliferative Rate
Non-Functional PNET (NF-PNET) 1,2
- The Majority of PNETs are Nonfunctional (60-90%)
- Can Secrete Substances but Do Not Present with Hormonal Syndromes
- Ex: Chromogranin, Pancreatic Polypeptide, Ghrelin, etc.
- Generally Present Later with a More Indolent and Protracted Course
- Symptoms are Generally Related to Mass Effect:
- Abdominal Pain
- Weight Loss
- Anorexia
- Nausea and Vomiting
- Obstructive Jaundice
Functional PNET (F-PNET)
- Insulinoma – *See Insulinoma
- Gastrinoma – *See Gastrinoma and Zollinger-Ellison Syndrome
- Glucagonoma – *See Glucagonoma and Glucagonoma Syndrome
- VIPoma – *See Vasoactive Intestinal Peptide-Secreting Tumor (VIPoma)
- Somatostatinoma – *See Somatostatinoma
- Other Less Common Functional PNETs:
- ACTH-Secreting Tumors (Cushing Syndrome)
- Serotonin-Secreting Pancreatic Neuroendocrine Tumor (Carcinoid Syndrome)
- PTHrp-oma (Mimics Hyperparathyroidism with Hypercalcemia)
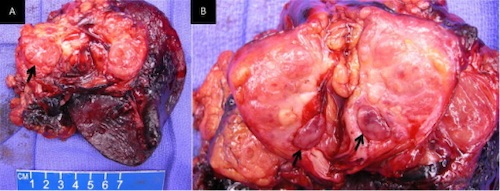
Resection of a Nonfunctional PNET 3
Functional PNET Comparisons
| PNET | Percentage of All | General Presentation |
| Insulinoma | 35-40% | Whipple’s Triad
|
| Gastrinoma | 16-30% | Peptic Ulcer Disease |
| Glucagonoma | < 10% | 4-D Syndrome
|
| VIPoma | < 10% | WDHA Syndrome
|
| Somatostatinoma | < 5% |
|
Size and Malignancy
- Most are Large (> 3 cm) and Malignant (60-90%) 5-8
- 75% Present as Advanced Disease
- Conversely, Insulinomas are Generally Small (< 3 cm) and Benign (93%) 9,10
Association with Multiple Endocrine Neoplasia Type 1 (MEN-1)
- Most are Sporadic
- Insulinoma: 6% 11
- Gastrinoma: 20-30% – The Most Common PNET Associated with MEN-1 12
- Glucagonoma: 3-10% 6,13,14
- VIPoma: 5% 15
- Somatostatinoma: 35-45% 16
- Although MEN-1 is Common in Somatostatinoma, Somatostatinoma is Overall One of the Least Common PNETS in MEN-1 (< 1%) 16
Most Common Location in the Pancreas Mn
- Insulinoma: Even Distribution Throughout the Pancreas 17
- Gastrinoma: Head 18
- Glucagonoma: Body and Tail 19
- VIPoma: Body and Tail 20
- Somatostatinoma: Head 8
Staging
TNM Staging (AJCC 8) 21
| T | N | M | |
| 1 | Limited to the Pancreas (< 2 cm) | Any Regional Lymph Node Involvement | M1a – Liver |
| 2 | Limited to the Pancreas (2-4 cm) | ||
| 3 | Limited to the Pancreas (> 4 cm) or Invades the Duodenum/CBD | ||
| 4 | Invades Adjacent Organs or Large Vessels |
- For T Stage – Multiple Tumors are Designated as T(#) (Example: T3(4))
- If Number or Tumors is Too Numerous or Unavailable – T(m)
- *Note: This System Does Not Apply to High-Grade, Poorly Differentiated Neuroendocrine Carcinoma (PNEC) – These are Staged as an Exocrine Pancreatic Cancer
TNM Stage 21
| Stage | T | N | M |
| I | T1 | N0 | M0 |
| II | T2-3 | N0 | M0 |
| III | T4 | N0 | M0 |
| Any T | N1 | M0 | |
| IV | Any T | Any N | M1 |
WHO 2010 Grading 22
| Grade | Differentiation | Ki-67 Index | Mitotic Count (/10 HPF) |
| G1 (Low) | Well | ≤ 2% | < 2 |
| G2 (Intermediate) | Well | 3-20% | 2-20 |
| G3 (High) | Poorly | > 20% | > 20 |
Diagnosis
Diagnosis
- Insulinoma: High Insulin Levels During an Episode of Hypoglycemia 23
- Gastrinoma: High Fasting Serum Gastrin (FSG) and Measurement of Gastric pH 24
- Secretin Stimulation Test if Initial Findings are Not Diagnostic
- *See Gastrinoma and Zollinger-Ellison Syndrome
- Glucagonoma: High Fasting Glucagon 25
- VIPoma: High Plasma VIP 26
- Somatostatinoma: High Fasting Plasma Somatostatin 27
- May Be Made by Biopsy/Histology if Classic Presentation is Absent
- *See Somatostatinoma
Localization
- Initial Imaging: Noninvasive (CT or MRI)
- Somatostatin Receptor Imaging
- Consider if Initial Imaging Fails to Localize
- Options:
- Somatostatin (Octreotide) Receptor Scintigraphy (SRS) – Classic Test Used
- Functional PET Scan (Ga-68 DOTATATE) – Becoming More Prevalent with Higher Sensitivity
- Insulinomas Demonstrate Relatively Low Somatostatin Receptor Expression (May Be More Difficult to Detect than Other PNETs) 28,29
- If Noninvasive Imaging Fails: Invasive Imaging
- Endoscopic Ultrasound (EUS) – Generally Preferred Next Step
- Selective Arterial Stimulation with Hepatic Venous Sampling
- Use: Insulinoma (Calcium Stimulation) or Gastrinoma (Secretin Stimulation)
- Selective Visceral Angiography
- For Gastrinoma, Consider Surgical Exploration with Palpation or Intraoperative Ultrasound if High Suspicion but All Imaging Negative
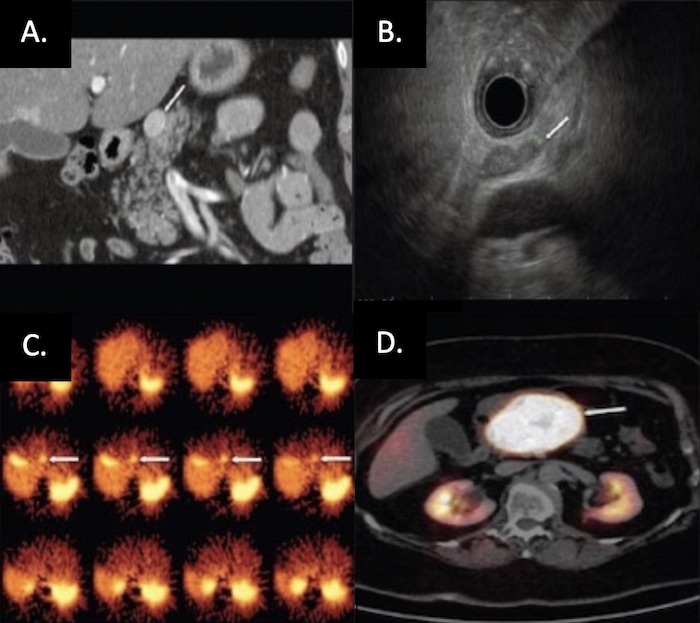
PNET on Imaging: (A) CT, (B) EUS, (C) SRS, (D) Functional PET 30
Treatment
Surgical Resection (Treatment of Choice)
- Small (< 2-3 cm): Enucleation
- *Enucleation is Controversial for the More Malignant PNETs (VIPoma, Somatostatinoma, or Glucagonoma)
- Large (> 2-3 cm): Surgical Resection
See Individual Pages for Specifics on Management
- Insulinoma – *See Insulinoma
- Gastrinoma – *See Gastrinoma and Zollinger-Ellison Syndrome
- Glucagonoma – *See Glucagonoma and Glucagonoma Syndrome
- VIPoma – *See Vasoactive Intestinal Peptide-Secreting Tumor (VIPoma)
- Somatostatinoma – *See Somatostatinoma
Pancreatic Neuroendocrine Carcinoma (PNEC)
- Poor Prognosis with Rapid Disease Progression
- Resectable Disease: Surgical Resection with Adjuvant Chemotherapy
- Unresectable Disease: Palliative Chemotherapy
Mnemonics
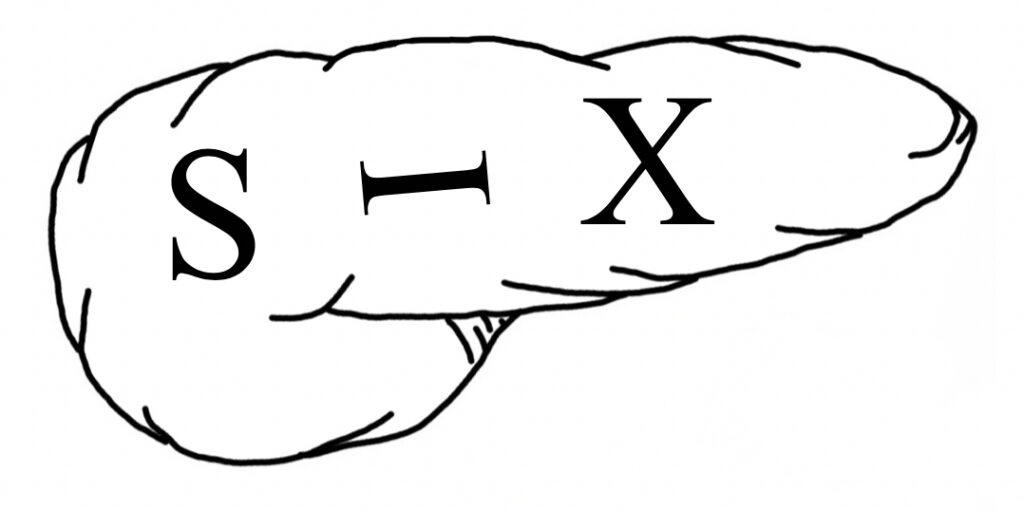
Most Common Site of PNET’s 31
- “6: The Number of the Pancreas”
- Pancreas Looks Like a #6
- *Used in Other Mnemonics: Pseudocyst and Chronic Pancreatitis
- “SIX” Written on Pancreas:
- “S” in Head: gaStrin and SomatoStatin
- “I” Anywhere: Insulin
- “X” in Body/Tail: Glucagon and VIP

References
- Cloyd JM, Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J Gastroenterol. 2015 Aug 28;21(32):9512-25.
- Dumlu EG, Karakoç D, Özdemir A. Nonfunctional Pancreatic Neuroendocrine Tumors: Advances in Diagnosis, Management, and Controversies. Int Surg. 2015 Jun;100(6):1089-97.
- Rodriguez RA, Overton H, Morris KT. Pancreatic neuroendocrine tumor with splenic vein tumor thrombus: A case report. Int J Surg Case Rep. 2014;5(12):1271-4. (License: CC BY-NC-ND 3.0)
- McKenna LR, Edil BH. Update on pancreatic neuroendocrine tumors. Gland Surg. 2014 Nov;3(4):258-75. doi: 10.3978/j.issn.2227-684X.2014.06.03.
- Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006 Nov;85(6):295-330.
- Marcal LP, et al. Pancreatic Neuroendocrine Tumors. Oncologic Imaging: A Multidisciplinary Approach, 2e. 2023.
- Una Cidon E. Vasoactive intestinal peptide secreting tumour: An overview. World J Gastrointest Oncol. 2022 Apr 15;14(4):808-819.
- Nesi G, Marcucci T, Rubio CA, Brandi ML, Tonelli F. Somatostatinoma: clinico-pathological features of three cases and literature reviewed. J Gastroenterol Hepatol. 2008 Apr;23(4):521-6.
- Service FJ, McMahon MM, O’Brien PC, Ballard DJ. Functioning insulinoma–incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc. 1991 Jul;66(7):711-9.
- Pasieka JL, McLeod MK, Thompson NW, Burney RE. Surgical approach to insulinomas. Assessing the need for preoperative localization. Arch Surg. 1992 Apr;127(4):442-7.
- Placzkowski KA, Vella A, Thompson GB, Grant CS, Reading CC, Charboneau JW, Andrews JC, Lloyd RV, Service FJ. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987-2007. J Clin Endocrinol Metab. 2009 Apr;94(4):1069-73.
- Jensen RT, Fraker DL. Zollinger-Ellison syndrome. Advances in treatment of gastric hypersecretion and the gastrinoma. JAMA. 1994 May 11;271(18):1429-35.
- Castro PG, de León AM, Trancón JG, Martínez PA, Alvarez Pérez JA, Fernández Fernández JC, García Bernardo CM, Serra LB, González González JJ. Glucagonoma syndrome: a case report. J Med Case Rep. 2011 Aug 22;5:402.
- Lévy-Bohbot N, Merle C, Goudet P, Delemer B, Calender A, Jolly D, Thiéfin G, Cadiot G; Groupe des Tumeurs Endocrines. Prevalence, characteristics and prognosis of MEN 1-associated glucagonomas, VIPomas, and somatostatinomas: study from the GTE (Groupe des Tumeurs Endocrines) registry. Gastroenterol Clin Biol. 2004 Nov;28(11):1075-81.
- Parbhu SK and Adler DG: Pancreatic neuroendocrine tumors: Contemporary diagnosis and management. Hosp Pract 1995. 44:109–119. 2016.
- Garbrecht N, Anlauf M, Schmitt A, Henopp T, Sipos B, Raffel A, Eisenberger CF, Knoefel WT, Pavel M, Fottner C, Musholt TJ, Rinke A, Arnold R, Berndt U, Plöckinger U, Wiedenmann B, Moch H, Heitz PU, Komminoth P, Perren A, Klöppel G. Somatostatin-producing neuroendocrine tumors of the duodenum and pancreas: incidence, types, biological behavior, association with inherited syndromes, and functional activity. Endocr Relat Cancer. 2008 Mar;15(1):229-41.
- Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K. Diagnosis and management of insulinoma. World J Gastroenterol. 2013 Feb 14;19(6):829-37.
- Stabile BE, Morrow DJ, Passaro E Jr. The gastrinoma triangle: operative implications. Am J Surg. 1984 Jan;147(1):25-31.
- John AM, Schwartz RA. Glucagonoma syndrome: a review and update on treatment. J Eur Acad Dermatol Venereol. 2016 Dec;30(12):2016-2022.
- Soga J, Yakuwa Y. Vipoma/diarrheogenic syndrome: a statistical evaluation of 241 reported cases. J Exp Clin Cancer Res. 1998;17(4):389–400.
- Bergsland EK, Woltering EA, Rindo G. Neuroendocrine tumors of the pancreas. In: AJCC Cancer Staging Manual, 8th ed, Amin MB (Ed), AJCC, Chicago 2017. p.407. Corrected at 4th printing, 2018.
- Rindi G, Arnold R, Bosman F. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification of Tumours of the Digestive System., editor. 4th ed.Lyon: IARC Press; 2010.
- Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K. Diagnosis and management of insulinoma. World J Gastroenterol. 2013 Feb 14;19(6):829-37.
- Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: Increasingly difficult. World J Gastroenterol. 2012;18:5495–5503.
- de Herder WW, Hofland J. Glucagon & Glucagonoma Syndrome. 2023 Apr 7. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext.
- Schizas D, Mastoraki A, Bagias G, Patras R, Moris D, Lazaridis II, et al. Clinicopathological data and treatment modalities for pancreatic vipomas: a systematic review. J buon. 2019;24(2):415–23.
- de Herder WW, Hofland J. Somatostatinoma. 2023 Apr 12. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext.
- Portela-Gomes GM, Stridsberg M, Grimelius L, Rorstad O, Janson ET. Differential expression of the five somatostatin receptor subtypes in human benign and malignant insulinomas – predominance of receptor subtype 4. Endocr Pathol. 2007 Summer;18(2):79-85.
- Peltola E, Vesterinen T, Leijon H, Hannula P, Huhtala H, Mäkinen M, Nieminen L, Pirinen E, Rönty M, Söderström M, Arola J, Jaatinen P. Immunohistochemical somatostatin receptor expression in insulinomas. APMIS. 2023 Apr;131(4):152-160.
- Kartalis N, Mucelli RM, Sundin A. Recent developments in imaging of pancreatic neuroendocrine tumors. Ann Gastroenterol. 2015 Apr-Jun;28(2):193-202. (License: CC BY-NC-SA 3.0)
- Velez DR. Mnemonic for the Most Common Locations of Functional PNET. The Operative Review of Surgery. 2023.

